| Jul 19, 2023 | |
The promise of liquid crystals for biomedicine |
|
| (Nanowerk Spotlight) Liquid crystals (LCs) are a distinct state of matter exhibiting properties between crystalline solids and isotropic liquids. Their partially ordered structure gives rise to unique characteristics that have recently shown promise for diverse biomedical applications. | |
| LCs can be categorized based on their formation mechanism. Thermotropic LCs form as temperature changes. They transition from the solid state to the LC state at the melting point as crystals melt, and from the LC state to an isotropic liquid state at the clearing point as order is lost with increasing temperature. Lyotropic LCs require the presence of solvents to form. They consist of amphiphilic molecules like lipids that self-assemble into nanostructured phases like hexagonal, cubic, and lamellar at certain concentrations, driven by hydrophobic and hydrophilic interactions. | |
| Components that make up LCs range from biological materials like DNA, cellulose, collagen, chitin and silk fibers, to synthetic mesogenic molecules, polymeric liquid crystals, and nanomaterials like carbon nanotubes and graphene oxide sheets. | |
| LCs can arrange into phases with different levels of order, such as the nematic phase where rod-shaped molecules are aligned but not positionally ordered, the smectic phase with a layered structure, and the chiral nematic or cholesteric phase where molecules are arranged in twisted helical layers that selectively reflect light. | |
 |
|
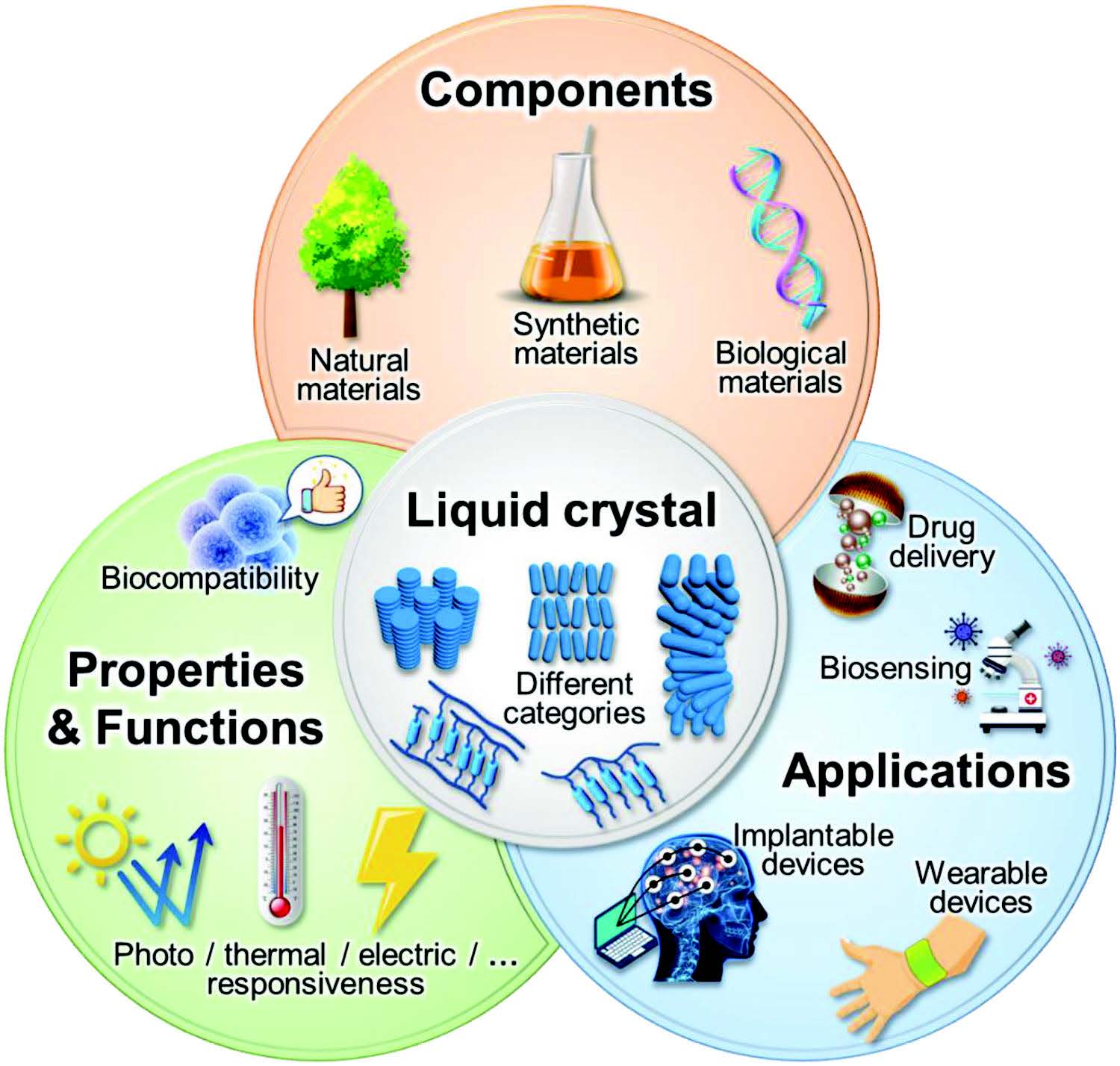
| Overview of liquid crystal materials for biomedical applications. (Reprinted with permission by Wiley-VCH Verlag) | |
| A recent review article in Advanced Materials ("Liquid Crystal Materials for Biomedical Applications") summarizes the latest progress of LC materials for biomedical applications as shown in the above illustration. | |
| Several key properties of LCs underlie their promise for biomedical use. LCs exhibit optical anisotropy and birefringence due to differences in refractive index parallel and perpendicular to the director axis. This allows control over light transmission and reflection. | |
| Cholesteric LCs produce brilliant structural colors due to periodic helical twisting. This makes LCs useful as bioimaging agents and optical reporters. LCs also have directionally dependent thermal conductivity, with higher in-plane versus out-of-plane heat transfer. This allows precise control over thermal pathways in LC materials. | |
| Another important feature is their stimuli-responsiveness – LCs can change ordering or phase transition in response to temperature, light, electric or magnetic fields. This enables reversible transitions between opaque and translucent states, or nematic and isotropic phases. | |
| Further, polymeric LCs can demonstrate actuation and macroscopic shape changes driven by alterations in molecular order. This is highly advantageous for soft robotics applications mimicking biological muscle movements. With rational molecular design, LCs can be engineered to be biocompatible and biodegradable for safe interaction with human tissues. | |
| One major biomedical application area for LCs is in advanced drug delivery systems. Lyotropic LC nanostructures such as hexosomes and cubosomes provide high loading capacity for hydrophobic, hydrophilic and amphiphilic drugs due to their compartmentalized internal domains. LC delivery vehicles protect sensitive drugs from degradation in transit and allow for sustained or triggered release at target sites. By tuning LC phase transition behavior using temperature or pH, researchers have developed smart systems for controlled drug release. LCs have also been used to safely encapsulate and deliver fragile genetic materials like DNA and RNA, holding great promise for gene therapy. | |
| LCs are also promising as bioimaging agents and contrast enhancers. Incorporating magnetic nanoparticles within LCs creates MRI contrast agents with enhanced relaxivity compared to free nanoparticles. Lyotropic LC nanoparticles made from food-grade lipids and loaded with nitroxide radicals have been used for T1-weighted MRI in animal models. Fluorescent lyotropic LC nanocarriers have also been developed for cell imaging and tracking applications. The nanostructured domains help confine and stabilize the imaging payloads. | |
| In the field of tissue engineering, LC polymer networks and hydrogels have been explored as dynamic scaffolds to support cell adhesion, growth and differentiation. The orientational order and anisotropy of LCs can direct the alignment of cells along the director axis, which is useful for engineering highly oriented tissues like muscle and nerve. By using light-responsive LCs, researchers can spatiotemporally control surface topography and mechanics at the microscale, enabling regulation of cell migration patterns. This helps replicate key features of the native extracellular matrix environment. | |
| The chemical stability, thermal durability, and tailored mechanical properties of LC polymers make them useful substrate materials for implantable biomedical devices. LCs have been microfabricated into substrates for flexible and lightweight microelectrode arrays able to record high-fidelity neural signals. LC polymers have also shown promise for the packaging of retinal and cochlear visual/auditory implants due to moisture resistance surpassing conventional polymers. However, improvements in optical transparency need to be made for enhanced device performance. | |
| LC biosensors provide a label-free approach to detecting target biomolecules through the optical signals generated when analyte binding alters the orientation of the LC matrix. LC-solid interfaces on patterned substrates have enabled highly sensitive detection of nucleic acids and proteins down to picomolar levels by observing changes in birefringent appearance. Similarly, LC-water interfaces functionalized with recognition elements have been designed for detection of disease biomarkers and bacterial contamination based on reorientation of LCs at the interface. | |
| Lastly, wearable LC devices offer a versatile platform for visual sensing a wide variety of chemical and physical stimuli. Cholesteric LC films can display reversible and quantifiable color changes in response to mechanical strain, temperature, humidity and biomarkers by tuning the helical pitch. Research teams have developed customizable LC-based sensor patches that conformally monitor human motions and health indicators like glucose levels in sweat. With further development, wearable LC sensors may provide continuous and non-invasive health monitoring to improve diagnostics and therapeutic interventions. | |
| In summary, liquid crystals are an exciting class of functional soft matter exhibiting tunable structural order and fluidity. Interdisciplinary research over the past few decades has uncovered their immense potential for advancing biomedical technologies, from targeted drug delivery systems to high-performance biosensors. While challenges remain in optimizing LC biostability and biocompatibility for prolonged human use, these dynamic materials promise to benefit human health in the future thanks to their distinctive stimuli-responses and biologically relevant optical and mechanical properties that interface with living systems. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
