| Nov 02, 2023 | |
Bioengineered carbon nanotube biosensor allows ultrasensitive detection of disease biomarkers |
|
| (Nanowerk Spotlight) The rapid detection of disease biomarkers is critical for early diagnosis and timely treatment of illnesses. However, conventional methods like immunoassays require labelling and long processing times, making point-of-care testing difficult. An emerging alternative is nanomaterial-based electronic biosensors. These devices can directly convert biological signals into measurable electrical outputs compatible with microelectronics. This enables integration of amplification, processing and display functions for rapid, accurate and quantitative sensing. | |
| One promising nanomaterial is carbon nanotubes (CNTs), which have exceptional electrical properties and biocompatibility. CNT field-effect transistors (FETs) act as highly sensitive electronic switches that can detect minute changes upon binding of target analytes. However, functionalizing CNTs to specifically recognize biomarkers has been a major challenge. Non-ideal probe configurations and low binding efficiency restrict sensitivity and selectivity. | |
| To overcome these limitations, scientists have now developed an innovative “biomarker entrapment system” (BioES) based on CNT-FETs. Through synergistic nanomaterial and biomolecular engineering, the BioES demonstrates unprecedented sensitivity in identifying protein and DNA biomarkers linked to endometriosis, monkeypox and cancer. The research establishes a versatile sensor architecture compatible with different target analytes, paving the way for next-generation point-of-care diagnostics and high-throughput epidemic screening. | |
| The researchers first set out to tackle endometriosis, a chronic and often painful gynecological condition affecting up to 10% of women. A key challenge is detecting estrogen receptor beta (ERβ), a biomarker found to be significantly elevated in endometriotic lesions. Ultrasensitive identification of this protein could enable early diagnosis and better personalized treatments. However, conventional immunoassays are incompatible with rapid point-of-care testing. | |
| By engineering the BioES specifically for ERβ capture, the team achieved groundbreaking sensitivity along with clinical applicability. They reported their findings in Advanced Materials ("Multi-Body Biomarker Entrapment System: An All-Encompassing Tool for Ultrasensitive Disease Diagnosis and Epidemic Screening"). | |
 |
|
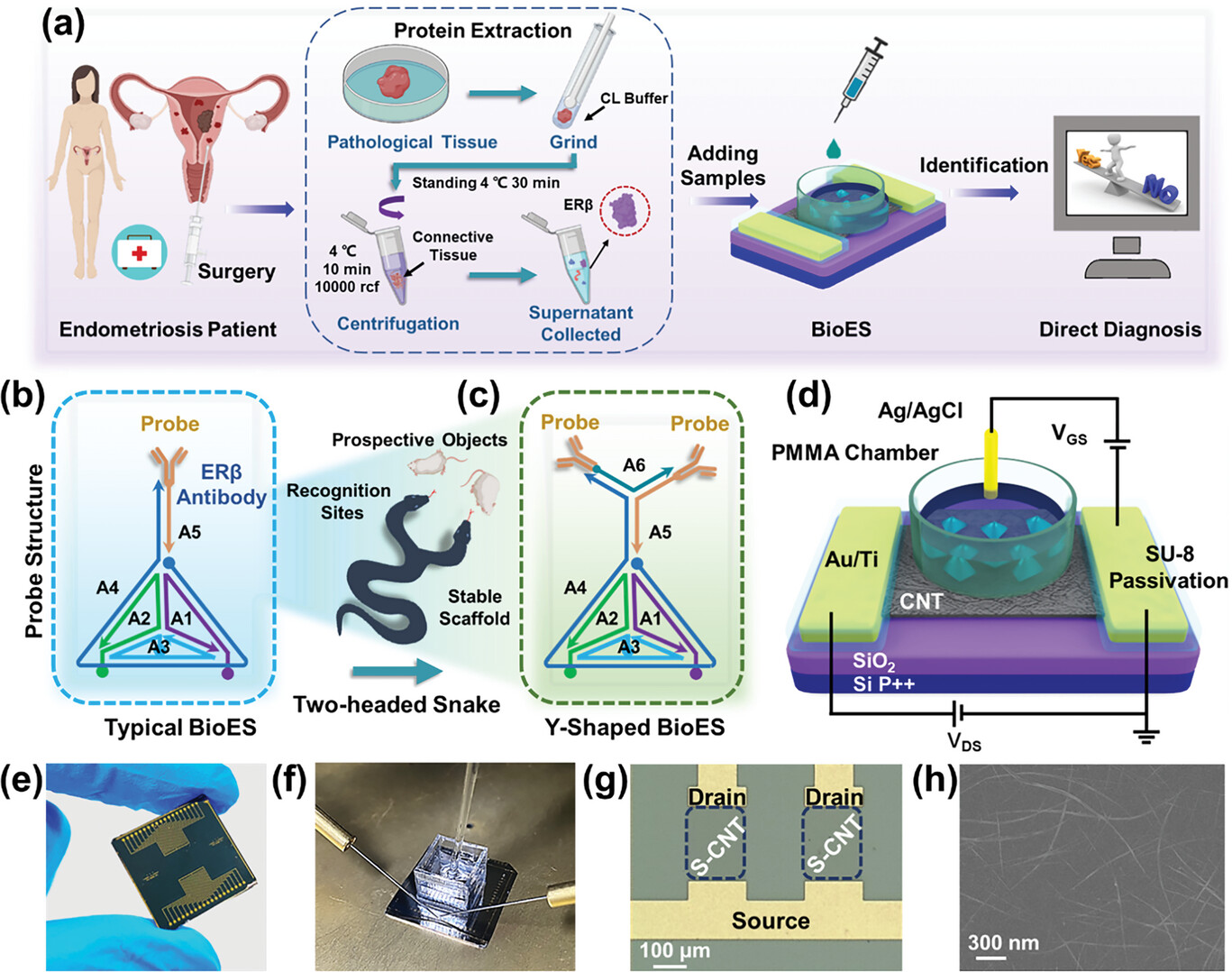
| Schematic diagram of the BioES. a) The workflow of endometriosis identification via the BioES. The Schematic diagrams of b) typical BioES c) Y-shaped BioES. d) The device configuration of EG-FETs. e) Diagram of the devices. f) The BioES testing platform. The EG-FETs are placed on the well-grounded Agilent B1500A. Ag/AgCl as the reference electrode is immersed in the PBS buffer. g) The top-view optical micrograph of EG-FETs. h) SEM image of S-CNT channel. (Image: Reprinted with permission by Wiley-VCH Verlag) | |
| The core of the BioES is an electrolyte-gated carbon nanotube transistor, which can directly convert biological signals into measurable electrical outputs. To enhance recognition, the scientists created a striking “multi-headed snake” architecture by decorating the nanotubes with a tetrahedral DNA framework tipped with ERβ antibodies, increasing target occupancy. | |
| This bioengineered configuration achieved an unprecedented ERβ detection limit of 6.74 attomoles per liter in cell lysates and accurately identified clinical endometriosis tissue samples. The team also optimized the design into a dual-head format which lowered the detection limit of the monkeypox antigen A35R to 991 attomoles per liter in serum. | |
| Moreover, when engineered with probes targeting tumor associated AKT2 DNA sequences, the BioES platform detected fragment copies down to 0.21 attomoles per liter in serum, demonstrating applicability in cancer screening. | |
| The researchers attribute the BioES’s ultrasensitivity to the rigid, compact structure of the DNA framework which reduces crowding and entanglement of recognition elements compared to conventional linear probes. The two-headed architecture also enables synergistic capturing of targets. | |
| The versatility of the BioES lies in its ability to detect diverse biomarkers by simple modification of the recognition sites. This could allow prompt adaptation to detect new viral mutations critical for epidemic control. Moreover, the BioES has a complementary metal-oxide-semiconductor compatible architecture enabling wafer-scale manufacturing and system-on-chip integration. | |
| The researchers envision the BioES could underpin next-generation “lab-on-a-chip” devices that screen for hundreds of diseases in minutes using only a fingerprick of blood. By seamlessly linking emerging biomarkers with microelectronics, the platform may realize the long-standing goal of highly multiplexed diagnostics. | |
| Additionally, the ultrasensitive antigen trap architecture could be leveraged to detect disease biomarkers in exhaled breath and other alternative specimens, facilitating non-invasive testing. Beyond diagnostics, the BioES could accelerate pharmaceutical research by facilitating high-throughput drug screening. | |
| Overall, this nanobiosensor innovation lays the foundation for point-of-care diagnostics meeting the demands of personalized medicine and public health surveillance in the post-COVID era. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
