| Nov 05, 2023 | |
New method for stable emulsions using engineered nanoparticle roughness |
|
| (Nanowerk Spotlight) Researchers have harnessed the unique properties of rough nano-scale particles to create new continuous emulsion channels with potential applications ranging from drug delivery to purification. Their work, published in Advanced Functional Materials ("Continuous Emulsion Channels Achieved by Controlling the Aqueous–Oil Interface Solely with Rough Colloids"), demonstrates how roughness-enhanced friction can slow dynamics and stabilize complex liquid-liquid interfaces. | |
| Emulsions that mix immiscible liquids are central to products from food to cosmetics. For instance, emulsions like mayonnaise mix liquids that don’t normally blend. They require added molecules called surfactants to stabilize the interface between the liquids. (Mayonnaise is an emulsion of oil, egg yolk, and either vinegar or lemon juice, with seasonings for flavor. The egg yolk acts as an emulsifier because it contains lecithin, a substance that helps to blend and stabilize the mixture of oil and the water-based vinegar or lemon juice.) | |
| Recently scientists have created emulsions using solid micro- or nanoscale particles instead of surfactants. The particles adsorb onto the liquid-liquid interface, locking it in place. | |
| These so-called Pickering emulsions can form discrete droplets. More intriguing are bicontinuous networks dubbed bicontinuous interfacially jammed emulsion gels or bijels. Rather than isolated blobs, bijels contain interlinked channels of the two liquids spanning the material. | |
 |
|
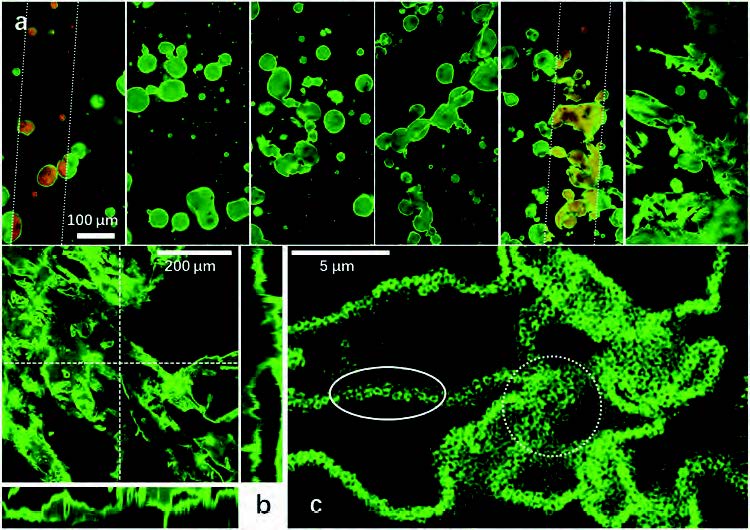
| The formation mechanism. a) The formation process of the continuous emulsion channels. From left to right: immediately after emulsification, 1, 3, 5, 7, and 10 min after emulsification. Alcohol soluble Eosin Y stainin solution has been used to label the ethanol (red fluorescence). b) Confocal microscopic observations prove the 3D characteristic of the continuous structures. c) Super-resolution fluorescence imaging technique (Stellaris 8, Leica, Germany) reveals that highly-curved interfaces are mainly supported by dense packed rough particles (marked in the dashed circle), while a flat interface can be stabilized by a monolayer (marked in the solid circle). (Reprinted with permission by Wiley-VCH Verlag) | |
| To date bijels have required a careful balance of liquids plus modifications to the particles’ surface chemistry. Scientists have now taken a new approach using rough silica particles without any chemical add-ons but with tailored nanoscale roughness. Their innovative approach sidesteps previous needs for bespoke surface chemistry modifications or precisely balanced fluid pairings. | |
| The researchers found these “bumpy” particles form unusual networks when mixed into blends of water, ethanol and silicone oil. The key breakthrough was using engineered roughness to manipulate the inter-particle and particle-liquid interactions. | |
| Smoother spherical particles cannot stably reinforce bijels' intricate fluid interfaces. But the team discovered specific rough particles frustrate the usual process of phase separation. This kinetic trapping during blending creates non-equilibrium networks rather than the expected separated phases. | |
| Advanced imaging and simulations revealed how the particles’ surface topography hinders their rearrangement at the interface. The surface projections interlock, resisting compression and shear. This configurational locking preserves the bijel structures over spans of millimeters for seconds to minutes. | |
 |
|
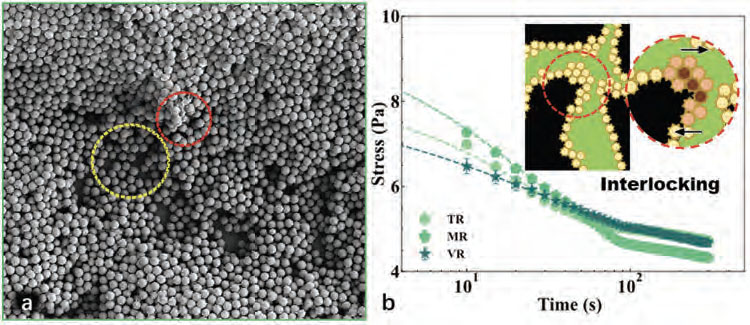
| Interlocking phenomenon. a) The transition from a percolated monolayer (marked in the yellow circle) to disordered accumulations (marked in the red circle) of MR particles at an air-water interface. b) Stress response upon an abrupt increase of shear rate for continuous channels stabilized by various rough particles. Dashed lines represent best fits of an empirical model.[21] For each surface roughness, at least three measurements were performed. (Inset of b)) An interlocking effect between rough particles forms force chains (marked in dark red) and other force-bearing aggregations (marked in light red) to provide mechanical support for the system. The arrows indicate that such interlocked aggregations are able to withstand further shearing forces. (Reprinted with permission by Wiley-VCH Verlag) | |
| Besides stabilizing the interface, networks of jammed rough particles impart solid-like mechanical rigidity. Such resistance to deformation differentiates bijels from other emulsions and enables applications like microreactor engineering. | |
| The researchers also analysed how the ethanol enabled bijel formation. It lowered surface tension to promote particle attachment at the oil-water boundary. And computational models showed preferential migration of ethanol from bulk water to further enrich the interface. This dynamic interfacial self-optimization was key for particle network formation. | |
| Surfactants can readily generate droplets, networks, and more. Uniquely this new rough nanoparticle approach opens routes to bijels using miscible and biocompatible liquid mixtures like ethanol-water and silicone oils. The researchers obtained consistent 3D networks across ~7mL volumes without any particle surface chemistry alterations. | |
| Such networks have potential uses from drug delivery to medical implants. As proof of concept the team loaded different cancer drugs into the separate fluid domains. Combinations exhibited enhanced efficacy over single drugs. | |
| The bijels also enabled removing contaminants from oil by trapping particles at the interface, demonstrating applications for purification and microfiltration. | |
| Critically, these insights on using engineered nanoscale roughness to frustrate phase separation have wider implications for emulsion design. The observations improve fundamental understanding of emulsification mechanisms. This could assist computational optimization of emulsions and other soft materials. | |
| By exerting fine control over fluid-fluid boundaries, the tailored particle approach may also enable emulsions with new architectural motifs. Further developing bijels as 3D microenvironments could open doors in synthetic biology, microfluidics, and materials development. | |
| Overall, this study shows nanoparticle physical aspects like roughness allow productively utilizing messy particle-interface physics. Combined with expanding capabilities to fabricate intricate particle shapes and surfaces, this promises more elaborate emulsion systems for research and industry. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
