| Nov 17, 2023 | |
Engineering multi-metal MOFs with customizable properties |
|
| (Nanowerk Spotlight) Over the past few decades, scientists have discovered that combining multiple metal elements into a single material can give rise to extraordinary new properties and functions. For example, specialized steel alloys made of iron and precise mixes of other metals have yielded steels that are both extremely lightweight and strong. This “multi-metal” mixing approach has shown great promise across materials science, giving researchers the power to fine-tune properties by artfully blending together different metals. | |
| But while the potential has been clear, realizing it has proven tremendously difficult in one burgeoning class of materials called metal-organic frameworks (MOFs). MOFs are a type of “crystal sponge” made by linking metals with organic molecules to form open, porous structures. Their customizable, sponge-like nature makes MOFs attractive for many cutting-edge applications, from energy storage to chemical sensing. | |
| The trouble has been that MOFs are very tricky to make from multiple metals. Attempts by researchers to mix different metals have often resulted in patchy, uneven distribution rather than uniform blending. This happens because different metals have varied reactivities, making them stubborn to uniformly mix together into the intricate, highly ordered crystal lattice of a MOF. | |
| But in a new study published in Advanced Functional Materials ("A Metal-Organic Framework Incorporating Eight Different Size Rare-Earth Metal Elements: Toward Multifunctionality À La Carte"), researchers report a breakthrough mixing technique that finally enables them to systematically blend many different metals into MOFs. Their approach relied on specially designed organic linker molecules that gently coax the different metal ingredients to uniformly mix within the MOF structure. | |
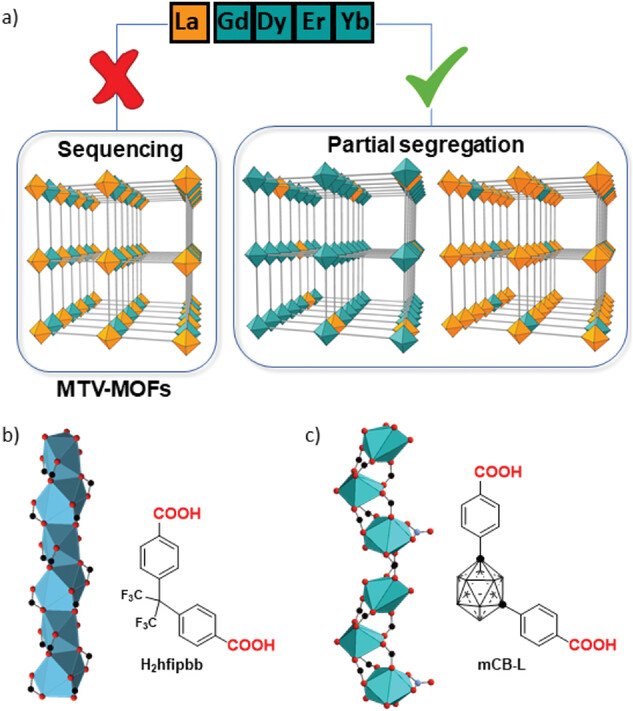 |
|
| Outcomes in the competitive formation of crystals with various bimetallic combinations and rod-shape secondary building units (SBU) examples. a) Combination of different size rare earth metal cations in rod-shaped SBUs constructed from dicarboxylated linkers results in preferential partial segregation. b) One rod SBU of RPF-4 composed of mono-metallic LnO9 polyhedra sharing faces (Ln = Yb or La) by H2hfipdd linker. c) one rod SBU of mCB-Tb composed of LnOx (x = 8–9) polyhedra sharing vertexes by mCB-L linker. | |
| “We demonstrate the synthesis of a multi-metallic MOF incorporating two, four, six or eight different rare-earth metal elements with different sizes and in nearly equimolar amounts and no compositional segregation,” explained study author Dr. José Giner Planas of the Institute of Materials Science in Barcelona. | |
| Rare-earth metals refer to chemical elements like neodymium, gadolinium and erbium that have unique magnetic and optical properties essential for technologies ranging from lasers to MRI scanners. By blending together eight different rare-earth metals, the researchers were able to custom-tune the thermal, optical and magnetic traits of the resulting MOF. | |
| The key was their specialized organic linker molecule, which contained molecular clusters called carboranes. Carboranes are exceptionally stable 3D carbon-boron molecules shaped like a soccer ball. In the study, the researchers connected two carborane balls together using a rigid rod-like linker. The bulky carborane balls gently pushed apart the different rare-earth metals, while the rod-shaped linker coaxed them to line up in a uniform mixed chain within the MOF. | |
| “The use of bulky linkers such as carboranes for the synthesis of multi-metal MOFs directs the formation of uniform metal chains, allowing the introduction of multiple cations with different sizes,” explained Dr. Planas. | |
| After systematically testing different rare-earth metal combinations, the team succeeded in blending together eight metals with different sizes—a first for MOFs. Analyses showed that the different metals were evenly distributed throughout the MOF crystal, rather than clumping together. | |
| Remarkably, the intricate MOF retained the distinct optical, magnetic and thermal traits contributed by the individual ingredient metals. “We demonstrate the co-existence of the eight metals in the MOF, and showcase the potentially tunable functionalities provided by the metals,” said Dr. Planas. | |
| For example, the MOF displayed versatile magnetic behaviors stemming from the different rare-earth metals. Two of the metals, terbium and dysprosium, endowed the MOF with “single-molecule magnet” behavior useful for data storage. At the same time, the metal gadolinium endowed it with a “magnetocaloric” effect that pumps heat in response to magnetism—an effect used in specialized nano-refrigerators. | |
| The findings show that specialized MOFs with highly tunable properties can be made to order by blending together different metals in precise ratios. According to Dr. Planas, this unlocks the possibility of creating “QuMOFs” using rare-earth metals known to have quantum properties prized for quantum computing. The carborane linkers could potentially be used to lay down the different rare-earth ingredients with atomic precision to make intricate quantum computer circuits embedded within a MOF. | |
| While much work remains, the metal-mixing technique provides researchers with a robust new platform for exploring multi-metal MOFs. Down the road, such mix-and-match MOFs could potentially yield a sweeping range of previously inaccessible applications by allowing scientists to dial in combinations of magnetic, optical, thermal and other traits simply by tuning metal ratios. The findings represent an important step toward the long-sought goal of designing tomorrow’s materials à la carte. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
