| Dec 12, 2023 | |
Easy-to-use, self-powered patch continuously tracks diabetes right under your skin |
|
| (Nanowerk Spotlight) Frequent blood draws are the uncomfortable reality for millions living with diabetes. These diagnostic finger pricks disrupt daily life and come with health risks like infection. Researchers have long sought a minimally invasive way to sample the clear fluid that surrounds our cells, known as interstitial fluid (ISF), which offers clues to blood sugar levels. | |
| Now, scientists report a promising advance - a small, wearable patch studded with tiny hollow needles that painlessly collect ISF for on-the-spot diabetes tracking. | |
| The field of microneedles, tiny needles about the width of a human hair, has progressed rapidly in recent years. But most microneedle patches still require cumbersome equipment like vacuum pumps or electrical stimulation for fluid extraction. The new research from Koç University in Turkey details a self-powered patch that needs only the press of a finger to start sucking up ISF. This simplicity comes from the integration of a vacuuming system made out of a stretchy polymer right on the patch itself. | |
| The findings have been published in Advanced Materials ("A Wearable Touch-Activated Device Integrated with Hollow Microneedles for Continuous Sampling and Sensing of Dermal Interstitial Fluid"). | |
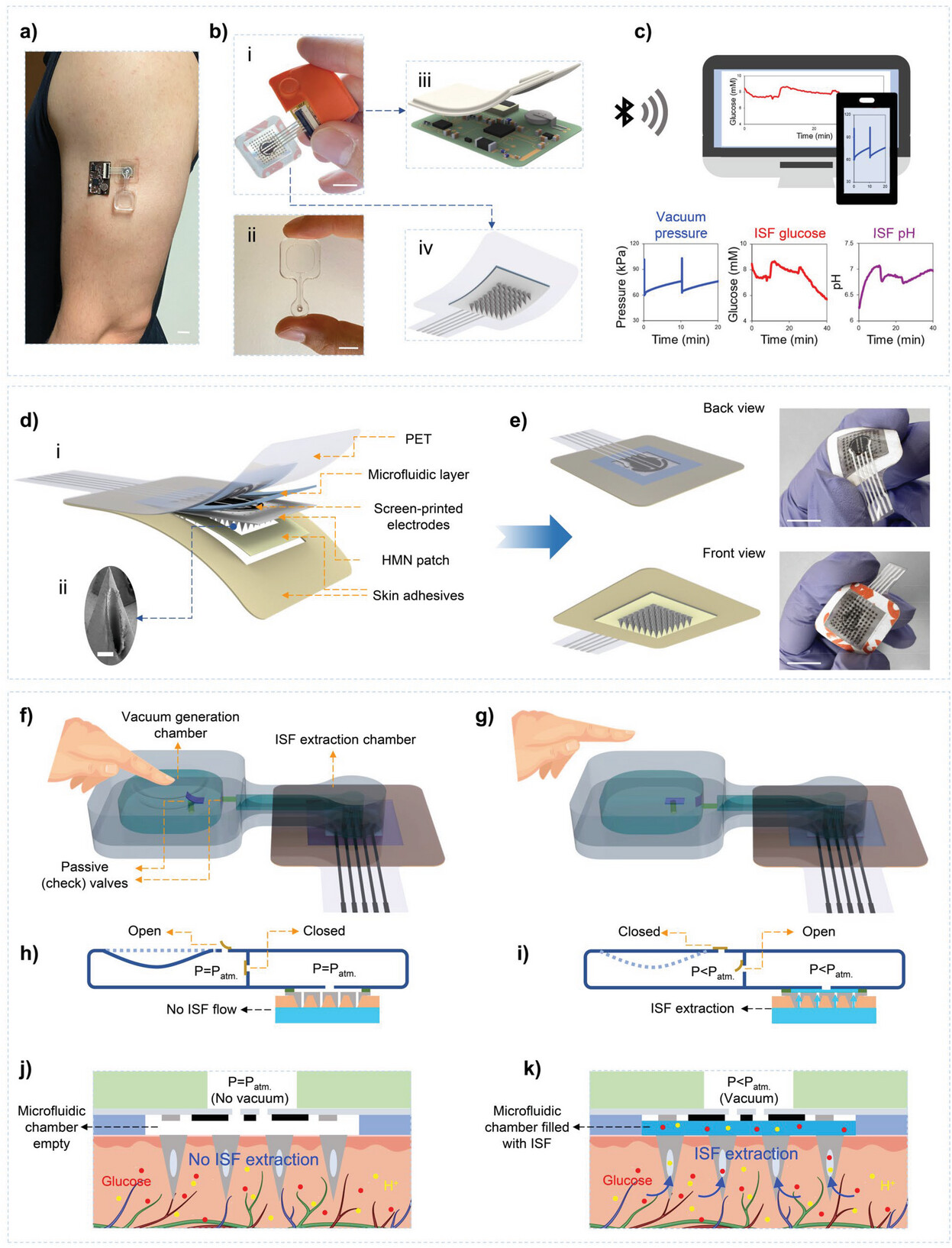 |
|
| Illustration of the solid hollow microneedles (HMN)-based wearable device for on-demand extraction of interstitial fluid (ISF) and electrochemical sensing. a) Photographic image of the developed device mounted on the human arm. b) Close-up images of different parts of the VIES system. (i) Integration of HMN patch and flexible electronic board. (ii) Image of PDMS-based vacuum generation device. Schematics of (iii) electronic board with encapsulation layer and (iv) HMN patch. c) Continuous monitoring of vacuum pressure and ISF glucose and pH levels. d) Schematic of HMN patch integrated with the microfluidic module. (i) The layer-by-layer layout of the HMN patch. (ii) SEM image of a single HMN fabricated by laser-drilling (scale bar: 200 µm). e) Back and front view of the HMN patch integrated with the microfluidic module. Schematics of the vacuum generation system f) upon applying and g) after removing the compressive force. Formation of the check valves h) upon applying and i) after removing the compressive force. Detailed illustration of the performance of the HMN-based VIES system, j) when there is no vacuum and k) after vacuum generation. (Scale bars for a, b, and e are 1 cm). | |
| “Such convenience and ease-of-use could allow continuous ISF sampling for diabetes management without disrupting normal activity,” said senior author Levent Beker, PhD. | |
| The tiny hollow microneedles themselves are crafted out of polycarbonate plastic. A laser carefully drills a hollow channel down the length of each 200 micrometer-wide needle. The patch tested in rats contained 25 needles in a 5 by 5 grid, each tall enough to penetrate through the outer layer of skin and reach the ISF-filled lower levels. Thanks to polycarbonate’s flexibility, the microneedles conform to skin curvature for uniform insertion across the array. | |
| Unlike metal alternative materials, the researchers confirmed polycarbonate’s mechanical resilience. The tiny needles retained their shape after repeated compression and puncturing tests. This durability prevents breakage under the skin, a drawback of some microneedle designs. | |
| The next step was integrating a vacuuming system to suck the fluid up through the hollow needles. The researchers molded a miniature chamber out of a silicone-based polymer called polydimethylsiloxane (PDMS). This rubbery material naturally rebounds to its original shape when released after squeezing. By attaching a one-way valve to an outlet nozzle, the scientists cleverly leveraged this elasticity to generate a sustained vacuum inside the chamber. Now, whenever the rebound pulls air molecules out of the chamber after a press, the valve closes to prevent backflow. By adding a second chamber and valve, finger pumps can continually strengthen vacuum pressure without resistance. | |
| “This furnished the necessary negative pressure for ISF sampling through the microneedles,” explained Beker. Liquid travels from the skin through to an outer compartment housing an embedded sensor array for analysis. Screen-printed electrodes customized to detect glucose and pH serve as a proof-of-concept but could be swapped for other disease biomarkers. | |
| The researchers first tested the patch by pressing it against samples of rodent tissue soaked in simulated ISF. Monitoring dye flow verified the vacuum system can extract fluid through the microneedles within minutes. Varying number of needles, pressure settings, and sampling times highlighted optimal collection conditions that maximize yield. Switching simulated ISF to known glucose and pH levels validated the integrated sensors pick up concentration changes. | |
| For true disease tracking capability, the technology needs to perform just as well extracting samples from a living creature. Application on rats demonstrated comparable functionality, effortlessly collecting microliter ISF volumes through finger-activated suction. Doubling needle numbers or enhancing vacuum strength increased yields even further. The only limitation noted was slightly dampened extraction rates over time versus tissue samples, likely due to natural skin barriers not replicated in the lab. Still, the microneedle device could reliably sense glucose and pH fluctuations in rat ISF. The wireless data transmission to a paired device means the system could integrate seamlessly into a smartwatch-style platform. | |
| “This self-powered patch allows simple, wearable ISF sampling and analysis without cumbersome tubing or equipment,” stated Beker. “Such convenience could truly unlock the promise of pain-free diabetes monitoring.” | |
| Of course, human trials remain to confirm efficacy across the diversity of skin types and health statuses. Integrating more biomarkers beyond glucose and pH would also widen diagnostic capabilities. Issues around occlusion and flow consistency through the tiny polycarbonate channels will need to be monitored over extended use. Still, by proving a reliable blood-free path to reading ISF, this microneedle technology lays vital groundwork for noninvasive disease tracking through the fluids already present in our skin. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
