| Jan 16, 2024 | |
Light-driven molecular jackhammers destroy cancer cells |
|
| (Nanowerk Spotlight) Scientists have long explored using light to precisely control molecular-scale machines inside living cells for applications like targeted drug delivery. However, progress in this promising field has been incremental. Early light-sensitive molecular actuators were structurally too simple to produce meaningful mechanical motions in complex intracellular environments. More intricate prototype systems were hampered by sluggish responses or a need for intense laser illumination. | |
| Now, researchers at Rice University have overcome prior barriers via sophisticated molecular engineering. By systematically evaluating an inventive class of motorized “molecular jackhammers,” the team elucidates key design principles to amplify these nanomachines’ capacity to perforate cell membranes using benign near-infrared light. | |
| This groundbreaking research represents a significant leap in molecular engineering. It not only overcomes previous limitations in light-activated molecular machines but also opens new horizons in targeted biomedical interventions. These molecular jackhammers, fine-tuned to respond to near-infrared light, mark a revolutionary step in the precise manipulation of cellular functions, paving the way for breakthroughs in cancer treatment and beyond. | |
| Additionally, the group formulates a “plasmonicity index” to mathematically predict jackhammer effectiveness based on their structures. This dual materials and computational framework promises to streamline optimization so molecular robots can swiftly and spatially-specifically disrupt cell membranes on-demand - clearing an exciting path for light-guided drug delivery, nano-surgery, and biosensing. | |
| The findings have been published in Advanced Materials ("How to Build Plasmon-Driven Molecular Jackhammers that Disassemble Cell Membranes and Cytoskeletons in Cancer"). | |
 |
|
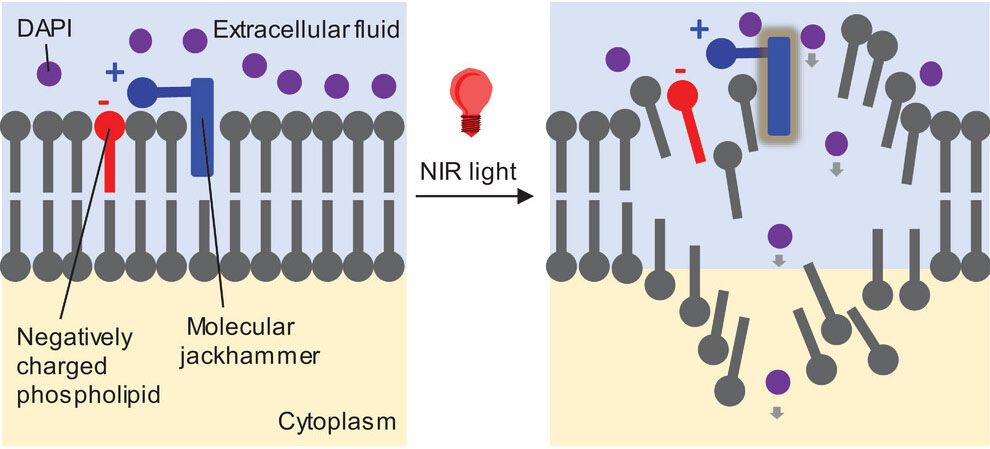
| Mechanistic pictorial model of vibronic-driven action to disassemble lipid bilayers. Step 1: Association of the molecular jackhammer to the lipid bilayer. Step 2: Activation of vibronic-driven action by NIR light to activate the molecular plasmons and vibrational modes in cyanine molecules. (Reprinted with permission by Wiley-VCH Verlag) | |
| The research taps into the emerging field of molecular plasmonics. When certain molecules absorb specific wavelengths of light, they enter an excited state where negatively charged electrons oscillate rapidly in unison, known as a molecular plasmon resonance. This resonance results in concerted molecular vibrations that can perform mechanical work, akin to a jackhammer. | |
| Previously, imaging techniques to observe these intricate molecular motions were lacking. However, this new study leverages confocal microscopy methods that let researchers view the disruptive effects of the molecular jackhammers on live cells, visually confirming their mechanical mechanism of action. | |
Overcoming Prior Roadblocks |
|
| Harnessing molecular plasmons for transformative applications like precision drug delivery has remained an elusive goal over the past decade. For example, plasmon-powered molecules tended to be inefficient or unstable when exposed to tissue environments inside the body. They were also notoriously tricky to design because their nano-mechanical behaviors relied on complex photophysical mechanisms that were arduous to predict or decipher experimentally. | |
| This new research tackles these prior roadblocks through systematic screens of 23 customized ‘molecular jackhammer’ molecules. This process elucidated which structural and photonic factors govern the jackhammers’ performance to permeabilize cell membranes. A structure-activity analysis also revealed which side chains bind the molecules most securely to targeted cell surfaces. | |
Ranking Performance with A “Plasmonicity Index” |
|
| To guide further improvement, the investigators developed a “plasmonicity index” that quantifies each molecules’ mechanical potential. Molecules were screened across solvents with different polarities, measuring how much their light absorption changed. Stronger shifts implied the molecules were highly sensitive to their surrounding environments. Mathematically, this earned them higher plasmonicity scores. | |
| The plasmonicity values tightly correlated with membrane-disruptive capacities measured through separate fluorescence assays. The best-performing molecular jackhammer, termed BL-204, packed intense mechanical power from infrared light to perforate over 50% of cancer cells at remarkably low concentrations under 0.2 micromolar. | |
| This plasmonicity concept now provides an invaluable metric to predict how modifications to photosensitive molecules will either heighten or dampen their light-activated nanomachine behaviors. It transforms the design process from a shot in the dark to a more quantitatively guided approach. | |
Catching Molecular Jackhammers in Action |
|
| Unlike previous studies where molecular motions could only be inferred indirectly through cellular effects, advanced fluorescence microscopy methods newly let researchers visualize the jackhammers’ mechanical havoc in real time. | |
| When triggered by brief two-minute pulses of infrared light, BL-204 molecules inflicted rapid destruction, tearing open pores in cell membranes. This allowed the entry of tracking dyes that stain nuclear DNA within minutes to confirm membrane rupture. Simultaneously, BL-204 jackhammering caused cell cytoskeletons to catastrophically contract as their structural integrity failed. The microscopic violence starkly contrasted cells exposed to infrared light alone, which activated natural motility programs to slowly move away. Control cells lacking BL-204 also continued functioning normally, proving BL-204’s mechanical effects depended on the synergistic combination of both the molecule and light. | |
Promising Therapeutic Potential Awaits |
|
| Looking forward, lead author Dr. Ciceron Ayala-Orozco notes “the ability to activate these molecular jackhammers with low intensity near-infrared light makes them appealing for future medical applications.” Near-infrared wavelengths can safely penetrate several centimeters through bodily tissues to trigger responses. Though not explored here, the group previously showed similar cyanine jackhammers could eradicate melanoma tumors in mice without signs of toxicity. | |
| The implications of this research are profound. It's not just about creating a new class of molecular machines; it's about redefining what's possible in non-invasive medical treatments. The Rice University team's innovation in harnessing near-infrared light for targeted cellular disruption could lead to unprecedented precision in drug delivery and cancer therapy, potentially transforming patient care. Such advancements underscore the immense potential of nanotechnology in revolutionizing medical science. | |
| That said, optimizing treatment safety remains paramount. An encouraging finding from the new paper was identifying modified molecular jackhammers with heightened anti-cancer capacities but reduced inherent toxicity without light activation. This improved “therapeutic index” lessens risks if accidental uptake occurred in healthy cells. Overall, armed with their new guiding design principles and evaluation toolkit, the Rice team believes they are now well-positioned to develop the next generation of light-powered nanomachines for drug delivery or pathogen elimination. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
