| Feb 13, 2024 | |
Researchers discover how functional nucleic acid nanoparticles can effectively deceive the immune system |
|
| (Nanowerk Spotlight) Since the discovery of the DNA double helix in 1953, researchers dreamed of leveraging nucleic acids for revolutionary biomedical applications benefiting human health. Early visionaries imagined engineering DNA and RNA as biosensors to detect toxins, vectors to enable gene therapies, and drug-loaded nanoparticles to precisely target medications. | |
| However, formidable barriers emerged. Once injected in animal models, synthetic nucleic acids provoked aggressive immune attack, breaking down before achieving therapeutic effect. High doses triggered inflammatory cascades or toxicity eliciting organ damage. | |
| Consequently, experts grew skeptical that nucleic acids held promise as technologies. But some persistent scientists explored new structural configurations and chemical modifications, slowly illuminating how to improve biocompatibility. | |
| By the 2000s, nucleic acids gained limited traction: RNA interference enabled experimental gene silencing and spherical nucleic acid nanoparticles exhibited improved drug delivery sans inflammation. Yet elongate, high aspect ratio nucleic acid nanofibers with enhanced functional capacity remained beyond reach. When introduced intravenously, these filaments instantly activated complement cascades tagging the foreign material for rapid elimination. | |
| Now in 2024, the decades-long quest to balance efficacy, safety and programmability of nucleic acid nanotechnologies (i.e. DNA nanotechnology and RNA nanotechnology) may have reached an inflection point. | |
| A research team, led by the Afonin Lab at UNC Charlotte, recently uncovered an elegant mechanism to regulate immune responses triggered by rod-like nucleic acid nanofibers upon delivery inside human cells. | |
| The team’s findings published in ACS Applied Materials & Interfaces ("Immunostimulation of Fibrous Nucleic Acid Nanoparticles Can be Modulated through Aptamer-Based Functional Moieties: Unveiling the Structure−Activity Relationship and Mechanistic Insights") detail a technique to camouflage immunostimulatory properties of nucleic acid nanofibrous scaffolds via DNA aptamer decoration, thereby expanding possibilities for employing such structures as vaccine adjuvants or immunotherapy vectors requiring controlled immune activation. | |
 |
|
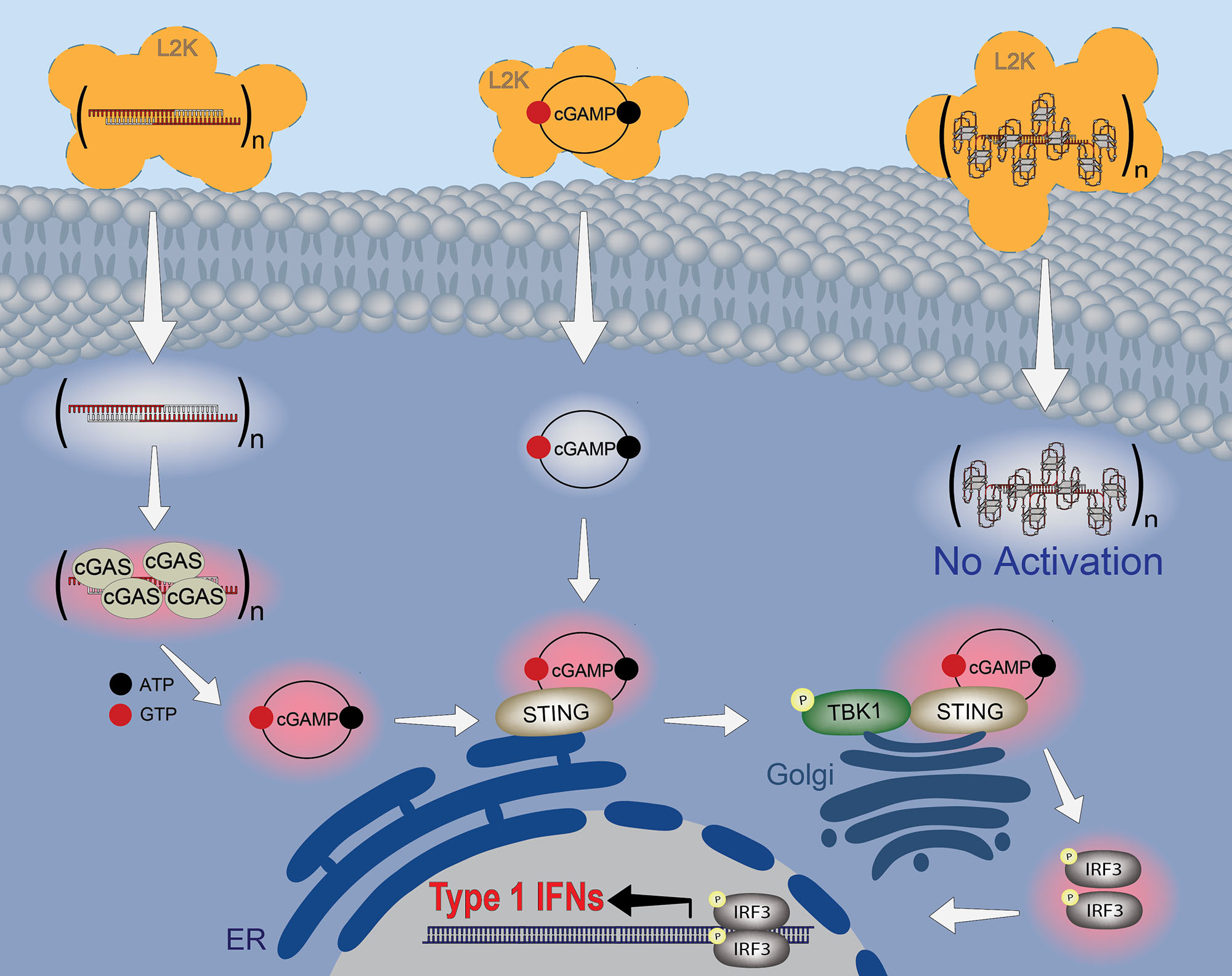
| Immunorecognition of RNA/DNA fiber NANPs via the cGAS-STING signaling pathway can be regulated through functionalization with aptamers. The summarizing schematic depicts trafficking and immunostimulation following treatment with nonfunctional fibers and positive control (cGAMP); and the absence of activation in response to fibers functionalized with four aptamers per repeating unit. Orange clouds represent Lipofectamine 2000 (L2K) used for the delivery of all fiber NANPs to the cells. (Reprinted with permission by American Chemical Society) (click on image to enlarge) | |
| This discovery fundamentally advances the field's ability to regulate immune responses triggered by synthetically engineered nucleic acids. By elucidating an elegant biomolecular mechanism centered around the innate immune sensor protein cGAS, the researchers have defined an engineering solution to camouflage the immunogenicity of rod-like nucleic acid nanofibers requiring intracellular delivery for function. | |
| Since initial discovery in the 1980s, synthetic RNA and DNA have been molded into custom 3D nanoarchitectures exhibiting desirable traits like improved in vivo circulatory capacity and biodistribution or increased molecular cargo loading efficiency compared to linear structures. Consequently, experts recognized immense application potential if proper regulation of undesirable inflammatory side-effects and nanoparticle toxicity could be reliably achieved. | |
| Early efforts in the 2000s sought to overcome complications related to nanoparticle-triggered oxidative stress and subsequent inflammatory cascades by focusing almost exclusively on spherical nucleic acid nanostructures. | |
| However, the advantageous physiology offered by high-aspect-ratio rod-like architectures remained tantalizing yet elusive. Within the last few years alone, ingenious DNA and RNA nanofibers have enabled rapid blood coagulation control, synaptic regeneration after neuronal injury, and record-breaking in vivo circulation times. | |
| But once internalized within cells, the elongated morphology and repetitive surface patterns seem to confuse the innate immune system into launching inflammation as if combatting a dangerous pathogen invasion when such a response proves wholly unnecessary and frankly detrimental. | |
| "We hypothesized that we could resolve this conundrum via a scaffold 'passivation' strategy – essentially camouflaging the underlying nucleic acid framework by decorating the entire surface with custom DNA aptamers so that the innate immune system no longer recognizes the material as foreign," Afonin tells Nanowerk. | |
| DNA aptamers comprise short single strands of synthetic oligonucleotides that fold into specific 3D conformations capable of binding target proteins with high selectivity and affinity. The researchers incorporated either one or two different DNA aptamers per repeating unit along the nanofibers, experimentally validating their hypothesis first in lab cell cultures and then via molecular simulations. | |
| They discovered that the cytosolic sensor protein cGAS (cyclic GMP-AMP synthase) seems primarily responsible for recognizing unprotected nucleic acid nanofibers and triggering downstream inflammation signaling thereafter. | |
| However, when densely functionalized with DNA aptamers, far fewer cGAS proteins appear able to bind the effectively hidden nucleic acid core. Correspondingly, cytokine production and interferon responses dropped significantly. Intriguingly, the DNA aptamer sequences themselves elicited no meaningful immune reaction - only the uncamouflaged nucleic acid frameworks alerted the cell’s defenses. | |
| Simulations provided critical atomistic resolution detailing how decoration with DNA aptamers interferes with and reduces binding of the cytosolic sensor protein cGAS (cyclic GMP-AMP synthase), which initiates inflammatory signaling cascades inside certain human cells when uncloaked nucleic acid structures breach the cell membrane. With cGAS stifled through surface aptamer conjugation, the barrel-shaped bundles of interconnected DNA helices structurally persist yet evade immune detection. Findings remained consistent across three distinct human blood donors’ cells. | |
| By elucidating this evasion strategy, researchers dispelled concerns that intracellular trafficking would inherently inflame antiviral immunity when employing functional nucleic acid nanofibers. | |
| Instead, virologists now foresee engineering adjuvants or vectors capitalizing on controlled, aptamer-tuned immune stimulation. Cancer immunotherapists similarly envision cytokine-augmenting nucleic acid nanorods to rouse anti-tumor immune responses. Both specialties plan to investigate if nucleic acid nanofilaments internally delivered via aptamer-liposome complexes might escape immediate lysosomal degradation and thereby enhance antigen presentation. | |
| Additionally, neuroscientists hope to leverage these fundamental discoveries to fashion extraordinarily elongated nerve-guiding conduits supporting accelerated regeneration following traumatic injuries. | |
| This breakthrough portends immense impact across biomedicine and ushers in a new generation of smarter, therapeutic nucleic acid nanotechnologies seamlessly interfacing with human biology. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
