| Mar 15, 2023 | |
Small but mighty - the vast potential of RNA nanotechnology |
|
| (Nanowerk Spotlight) Nucleic acids are becoming increasingly popular for assembling nanoscale structures, thanks to their programmable dimensionality and direct applications in the biological field. | |
| Despite the fact that DNA nanotechnology has been explored more deeply over a longer period of time, RNA nanotechnology offers complementary opportunities and much broader applications. Despite a chemical structure that is similar to DNA, RNA often adopts two- and three-dimensional conformations that are far more complex than its DNA counterpart. Analysis of the rapidly growing structural and sequence databases for RNA has revealed novel RNA structural motifs and RNA-RNA interactions that have inspired scientists to pursue RNA-based nanoengineering with emphasis on biomedical applications. | |
| Akin to DNA origami, researchers have also developed RNA origami folding techniques in order to build a larger library of functional RNA particles. Unlike existing methods for folding DNA molecules, RNA origamis are produced during their transcription by RNA polymerase and they simultaneously fold into pre-designed shapes. These features may allow designer RNA structures to be grown within living cells and used to organize cellular enzymes into biochemical factories. | |
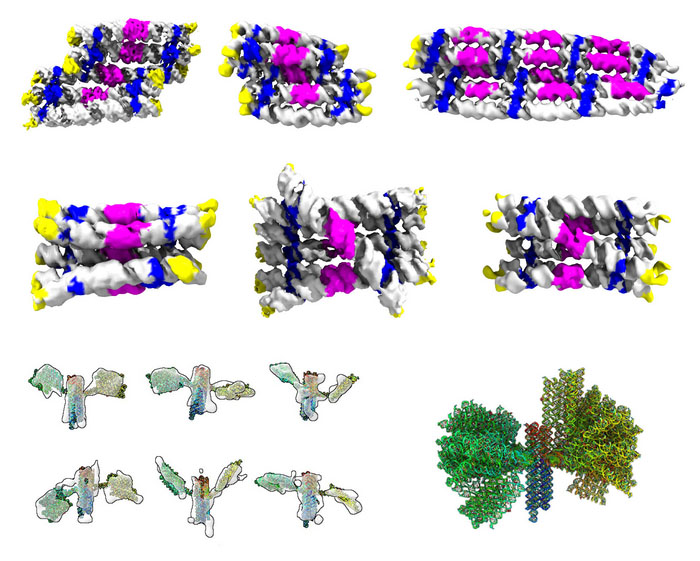 |
|
| Gallery of the different RNA origami structures that were determined by cryogenic electron microscopy and tomography. Top rows show structures of RNA rectangles and cylinders colored by RNA motifs. Bottom row shows structures of the nanosatellite colored in rainbow from 5’ to 3’ end (the reading direction of an RNA strand). Read more about this research. (Image: Ewan K. S. McRae, Aarhus University) | |
| In RNA nanoengineering, RNA molecules are the sole material used in the engineering process. However, researchers are also exploring alternative approaches to build more versatile nucleic acid nanoparticles, also known as NANPs, that involve the combined use of multiple materials, such as DNA, RNA, protein and even synthetic molecules. | |
| The growing research efforts in RNA nanotechnology have also translated into commercial progress, with the approval of three RNA interference (RNAi)-based drugs by the FDA and several RNA-based therapies currently in phase 3 clinical trials. | |
| RNA-focused biotechnology companies are continuously developing therapeutic nucleic acids with diverse mechanisms of action. The rapid development of COVID-19 mRNA vaccines is a prime example of the versatility of RNA. And just this month we saw reports that scientists have developed an mRNA-based vaccine that is 100% effective against the lethal plague bacterium. | |
Definition of RNA nanotechnology |
|
| RNA nanotechnology is an emerging field that combines the unique properties of RNA with the principles of nanotechnology to create new and innovative applications in medicine, biotechnology, and materials science. | |
| RNA stands for ribonucleic acid, a type of nucleic acid molecule found in cells that plays a key role in the process of gene expression. The chemical structure of RNA is very similar to that of DNA: each nucleotide consists of a nucleobase, a ribose sugar, and a phosphate group. There are two differences that distinguish DNA from RNA: 1) RNA contains the sugar ribose, while DNA contains the slightly different sugar deoxyribose (a type of ribose that lacks one oxygen atom), and 2) RNA has the nucleobase uracil while DNA contains thymine. Unlike DNA, most RNA molecules are single-stranded and can adopt very complex three-dimensional structures. | |
| RNA is involved in various biological processes, including protein synthesis, gene regulation, and the transmission of genetic information from one generation to the next, just to name a few. There are several types of RNA, including messenger RNA (mRNA), transfer RNA (tRNA), and ribosomal RNA (rRNA), each with their specific functions. In recent years, RNA has gained increasing attention as a versatile tool for engineering and manipulating biological systems at the molecular level. | |
| RNA nanotechnology offers several advantages over traditional biomedical and bionanotechnology approaches, such as greater specificity, biocompatibility, and flexibility in design. RNA nanoparticles, for example, can be designed to target specific cells or tissues, carry therapeutic payloads, and induce specific biological responses. These properties have made RNA nanotechnology a promising platform for drug delivery, diagnostics, gene editing, and gene therapy. | |
| More and more research groups worldwide are joining the blooming field of RNA nanotechnology – there are approximately some 100 research groups involved in this field – and developing novel approaches for the production of functional RNA nanoassemblies, which led to the recent establishment of the International Society of RNA Nanotechnology and Nanomedicine. | |
| Despite its potential, RNA nanotechnology is still a relatively young field, and several challenges and limitations need to be addressed before it can be widely adopted in clinical settings. These include issues related to stability, immunogenicity, and scalability, as well as ethical and regulatory considerations. | |
RNA nanoparticles for drug delivery |
|
| Traditional drug delivery systems face several challenges and limitations, such as low bioavailability, non-specific targeting, and toxicity. RNA nanoparticles offer several advantages over traditional drug delivery approaches, such as greater specificity, biocompatibility, and flexibility in design. | |
| The biggest challenge in developing a successful RNA therapy is its targeted delivery. That’s why recent advanced in RNA lipid nanoparticle engineering are so promising in overcoming this hurdle. For example, liver fibrosis has remained challenging to treat using RNA therapies due to a lack of delivery systems for targeting activated liver-resident fibroblasts. But just recently, researchers have demonstrated ligand-tethered lipid nanoparticles for targeted RNA delivery to treat liver fibrosis by delivering siRNA molecules right to the notoriously hard-to-target activated fibroblasts in the liver responsible for the buildup of collagen. | |
| In general, nucleic acid nanoparticles (NANPs) are a relatively novel type of nanomaterial that are exclusively made of rationally designed nucleic acids such as RNA, DNA and their chemical analogs. NANP technology is shaping up to become a very promising for targeted immunostimulation in human cells. | |
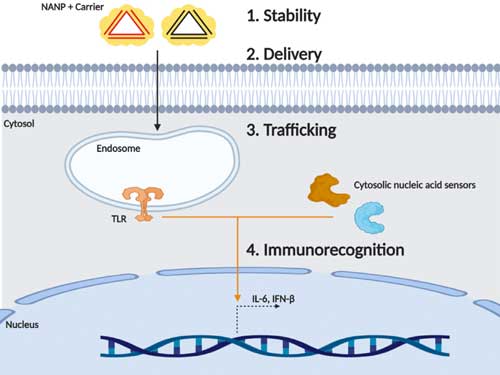 |
|
| The chemical composition of NANPs defines their physicochemical properties while tuning immune recognition and cellular compartmentalization. (Oxford University Press, Creative Commons CC BY) | |
| RNA nanoparticles can be designed to target specific cells or tissues, such as cancer cells, and deliver therapeutic payloads, such as small interfering RNAs (siRNAs), messenger RNAs (mRNAs) or antisense oligonucleotides, while avoiding undesired immune responses. These nanoparticles are designed to protect the nucleic acids from degradation and facilitate their efficient delivery to target cells or tissues. SiRNAs can silence specific genes that are responsible for disease, while mRNAs can produce specific proteins that can treat or prevent disease (as is the case with mRNA-based coronavirus vaccines). | |
| Several RNA nanoparticle-based drug delivery systems are currently in development or undergoing clinical trials. For example, researchers are exploring the use of RNA nanoparticles to deliver siRNAs for the treatment of cancer, viral infections, and genetic disorders. RNA nanoparticles have also been used to deliver mRNAs that encode therapeutic proteins, such as growth factors or enzymes, for the treatment of diseases such as hemophilia. | |
| RNA nanoparticles can also be designed to overcome the blood-brain barrier, which prevents many drugs from reaching the brain. Researchers are exploring the use of RNA nanoparticles to deliver drugs for the treatment of neurodegenerative diseases, such as Alzheimer's and Parkinson's disease. | |
RNA nanotechnology in diagnostics |
|
| Current diagnostic techniques often suffer from limitations such as low sensitivity, high false-positive rates, and the inability to detect disease at early stages. RNA nanotechnology offers several advantages for diagnostics, such as greater specificity and sensitivity. | |
| RNA nanoparticles can be designed to detect specific biomolecules, such as proteins or nucleic acids, that are indicative of disease. RNA aptamers, for example, are short RNA sequences that are selected to bind target molecules with high affinity and specificity. By attaching aptamers to RNA nanoparticles, researchers can create highly sensitive and specific diagnostic tools that can detect low levels of disease biomarkers. | |
| For example, during the SARS-CoV epidemic in 2002, researchers coated RNA aptamers with quantum dots to detect the nucleocapsid protein of the virus. The process was sensitive, rapid, and was done on a chip. | |
| NANPs can also be designed to perform a certain activity only upon detecting a specific signal. For instance, by interacting with a target strand or environmental variable of choice as a diagnostic step, switching NANPs can be designed to release therapeutics only when these interactions occur. | |
| Several RNA nanoparticle-based diagnostic tools are currently in development or undergoing clinical trials. For example, researchers are exploring the use of RNA aptamers attached to RNA nanoparticles to detect cancer biomarkers in blood samples. | |
RNA nanotechnology in gene editing and gene therapy |
|
| One example of RNA-based gene editing is RNA editing, which uses engineered enzymes to change specific nucleotides in RNA transcripts without altering the DNA sequence. This can potentially correct mutations that cause genetic diseases, such as retinal disorders. Another example is CRISPR-Cas13, which uses RNA-guided enzymes to target and cleave RNA molecules with high specificity and efficiency. This can potentially modulate gene expression, silence viral genes, or degrade pathogenic RNAs. | |
| Akin to drug delivery, RNA nanotechnology can also enhance gene therapy by improving the delivery and stability of nucleic acid therapeutics, such as siRNAs, miRNAs, antisense oligonucleotides, and mRNAs. | |
| These molecules can modulate gene expression by interfering with or replacing defective RNAs. However, they face challenges such as low bioavailability, rapid degradation, immune recognition, and off-target effects. RNA nanotechnology can overcome these challenges by designing nanostructures that protect, target, and release nucleic acid therapeutics in a controlled manner. | |
RNA nanotechnology in vaccine development |
|
| As witness by the phenomenal success of mRNA-based vaccines against SARS-CoV 2 virus, RNA nanotechnology with mRNA has started a new era in vaccinology. | |
| Traditional vaccine development involves the use of weakened or dead pathogens to stimulate the immune system to produce an immune response. However, RNA nanotechnology provides a novel approach to vaccine development by allowing for the direct delivery of rationally designed RNA molecules encoding antigens. | |
| RNA nanoparticles can be potentially designed to deliver mRNA molecules that encode specific viral or bacterial antigens. Upon delivery, these mRNA molecules are translated into protein antigens that trigger an immune response in the body, leading to the production of protective antibodies. | |
| One advantage of RNA nanoparticle-based vaccines is their exceptionally rapid development timeline. Unlike traditional vaccines, which can take several years to develop and manufacture, RNA nanoparticle-based vaccines can be designed and produced quickly – even within days. This makes RNA nanoparticle-based vaccines particularly useful in responding to emerging infectious diseases or pandemics. | |
| Diverse approaches to mRNA cancer vaccines, including dendritic cell vaccines and various types of directly injectable mRNA, have been employed in numerous cancer clinical trials, with some promising results showing antigen-specific T-cell responses and prolonged disease-free survival in some cases. | |
| Despite the promise of RNA nanoparticle-based vaccines, several challenges and limitations need to be addressed. These include issues related to stability, immunogenicity, and large-scale manufacturing. Depending on their architectures and design principles, RNA nanoparticles can be vulnerable to degradation by nucleases and may trigger unwanted immune responses. Manufacturing RNA nanoparticle-based vaccines at a large scale can also be challenging and costly. | |
Conclusion |
|
| In conclusion, RNA nanotechnology holds great promise in a variety of fields, including drug delivery, gene editing and therapy, and vaccine development. RNA nanoparticles can be designed with precise control over their structure and function, allowing for targeted and efficient delivery of therapeutic agents or antigen-encoding mRNA. | |
| While there are still challenges and limitations that need to be addressed, ongoing research and development in RNA nanotechnology is rapidly advancing the field. RNA nanoparticle-based therapeutics and vaccines have already shown great promise in clinical trials, and several products have received regulatory approval for use in humans. | |
|
Looking to the future, RNA nanotechnology is poised to revolutionize the field of medicine and transform the way we treat diseases. As we continue to learn more about the potential of RNA nanoparticles, we can expect to see even more groundbreaking applications in the near future.
|
|
Many thanks to Prof. Afonin at UNC Charlotte for proof-reading this text!
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
