| Posted: Feb 27, 2015 |
Ordered nanostructures from benzene could pave the way for novel nanotechnology applications
|
|
(Nanowerk News) A way to link benzene rings together in a highly ordered three-dimensional helical structure using a straightforward polymerization procedure has been discovered by researchers from RIKEN Center for Sustainable Resource Science and the University of Tokyo ("Aryne Polymerization Enabling Straightforward Synthesis of Elusive Poly(ortho-arylene)s"). “We expect our achievement to open up new areas of nanocarbon and materials science,” says Koichiro Mikami from the research team.
|
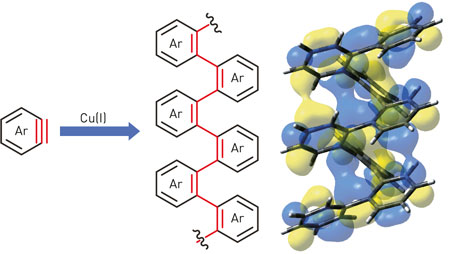 |
| Figure 1: Aryne (Ar) is reacted using a copper catalyst (Cu(I)) to assemble an ordered helical structure. (Image: K. Mikami, RIKEN Center for Sustainable Resource Science)
|
|
Benzene (C6H6) is the simplest of the wide range of ‘aromatic’ compounds, which have rings of carbon atoms surrounded by ‘delocalized’ electrons that circulate around the molecules. A long-standing challenge for chemists has been the development of a straightforward way to link rings of benzene together in a regular manner such that the carbon atoms are bonded directly to their neighbors in adjacent rings to form a structured material.
|
|
Masanobu Uchiyama from RIKEN and the University of Tokyo and his colleagues Mikami and Yoshihide Mizukoshi developed their linking procedure starting with the molecule aryne, which is very similar to benzene but has a triple bond between two adjacent carbon atoms in the ring (Fig. 1). After investigating many different combinations of chemicals and solvents, the researchers found a procedure involving copper ions that reliably links the rings together at the ortho position by a self-propagating polymerization reaction.
|
|
The product, called poly(ortho-phenylene), is the simplest member of wide range of possible poly(ortho-arylene)s that could carry a selection of different atoms or chemical groups in place of one or more of the hydrogen atoms on the benzene-derived rings. The researchers found that their poly(ortho-phenylene) molecules have a regular and highly ordered three-dimensional structure consisting of stacked six-carbon rings. Such highly ordered materials are precisely the kinds of chemical building blocks that might be put to good use as components for nanotechnology.
|
|
Having made this crucial breakthrough, the researchers’ next steps will be to explore the size and variety of structures that can be assembled by their technique. The team has so far managed to link approximately 100 rings together, but plans to extend this further and to control chain lengths and physical characteristics.
|
|
“We are exploring the electrical, photodynamic and thermal properties of these poly(ortho-phenylene)s toward creating novel electronic devices and liquid crystals,” explains Mikami. In addition to providing scaffolding structures, nanocompounds become most interesting when they can respond to light, electric fields or the presence of other chemicals in precise and useful ways. Mikami says the team is also looking at forming chemical cross-links between individual helical chains, extending the fabrication possibilities to another dimension.
|

