| Posted: Jul 25, 2016 |
Materials based on clusters of atoms may revolutionize the whole solar cell industry
(Nanowerk News) Lead-free, more efficient solar cells and other optoelectronics devices will likely be based on a family of materials known as hybrid perovskites. Scientists identified how to control different properties and stability in these solar cell materials using lead-free preparation.
|
|
These new design principles identified super-ion building blocks, clusters of atoms that carry the same charge as the ions that they replace. Scientists can tailor these building blocks improve stability and other desired traits (Journal of Materials Chemistry A, "Super-ion inspired colorful hybrid perovskite solar cells").
|
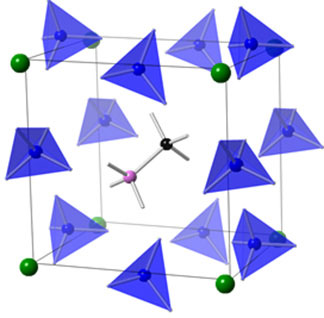 |
| The schematic illustrates a hybrid perovskite structure where the super halogens are the blue tetrahedrons, the metal atoms are green, and the alkali cation is in the middle (gray structure with pink and black atoms) (© The Royal Society of Chemistry).
|
|
These new design principles guided by simulations could lead to the next generation of solar cells and optoelectronics for lighting and data storage based on simple and environmentally friendly manufacturing methods.
|
|
Solar cell performance of hybrid perovskites has improved from under 4% efficiency in 2009 to over 20% efficiency today. However, perovskite stability still limits the performance. Also, hybrid perovskites commonly contain lead, which is toxic.
|
|
Now, researchers led by the Virginia Commonwealth University have used a multi-scale approach and a comprehensive study of over 40 materials to identify parameters and mechanism that control properties and stability in lead-free hybrid perovskites. Scientists performed simulations to fill the information gap in a series of lead-free hybrid perovskites, where experimental data were not available.
|
|
Computational methods included density functional theory, ab initio molecular dynamics simulations, and dynamic crystalline lattice calculations. The material can be pictured as a super crystal composed of super-ions, both super-alkali and super-halogen ions. Changing the halogen (chlorine, bromine, or iodine) and metal (germanium or tin) in the super ions affected their ionic radii and the ionic nature of the bonding.
|
| Scientists identified design principles that correlated the ionic nature of bonding to the electronic band gap and other photovoltaic-relevant properties. The team identified two methods to increase the ionic nature: using a smaller halogen to increase the radius ratio between super-ions and using a more metallic metal (tin compared to germanium). Also, they identified how the materials degrade when exposed to moisture and proposed counter strategies.
|
|
This new atomic-level understanding could lead to the development of more efficient and longer-lasting solar cells.
|

