| Posted: Dec 06, 2016 |
Biodegradable polymers made by chemical vapor deposition
(Nanowerk News) Polymerization by chemical vapor deposition (CVD) is a simple method for modifying surfaces by which topologically challenging substrates can be evenly coated with polymers. In the journal Angewandte Chemie ("Backbone-Degradable Polymers Prepared by Chemical Vapor Deposition"), researchers have now introduced the first CVD method for producing degradable polymers. Biomolecules or drugs can be attached by means of special side groups. This introduces new possibilities for applications like the coating of biodegradable implants.
|
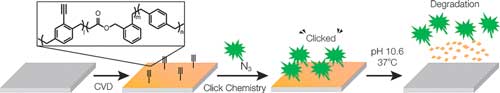 |
| Breaking the Backbone - Biodegradable polymers made by chemical vapor deposition. (© Wiley-VCH) (click on image to enlarge)
|
|
In CVD polymerization, the starting compounds are vaporized, activated at high temperature, and deposited onto surfaces, where they polymerize. In medical applications, substrates for implants are coated to allow functional groups to be added as anchors for the attachment of biomolecules or drugs.
|
|
Until now, however, it has only been possible to coat nondegradable implants, not materials that need to degrade after fulfilling their task, like surgical sutures, systems for controlled drug delivery, drug-eluding stents, or tissue engineering scaffolds. This is because it was previously not possible to make degradable coatings by CVD.
|
|
Scientists from the University of Michigan (Ann Arbor, USA), Northwestern Polytechnical University (Xi’an, China), and the Karlsruhe Institute of Technology (KIT, Eggenstein-Leopoldshafen, Germany) have now synthesized the first CVD polymer with a degradable backbone.
|
|
The research team led by Jörg Lahann succeeded by using two special types of monomer: The paracyclophanes usually used for this process were combined with cyclic ketene acetals. While the classic polymers based on paracyclophanes are connected exclusively through carbon–carbon bonds, the ketene acetal converts during the polymerization so that ester bonds (a bond between a carbon and an oxygen atom) are formed within the polymer backbone. Ester bonds can be broken in aqueous environments.
|
|
“The speed of the degradation depends on the ratio of the two types of monomer as well as their side chains,” explains Lahann. “Polar side chains make the polymer film less hydrophobic and accelerate degradation because water can penetrate more easily. The speed of degradation can thus be tailored to the intended use.” Tests with cell cultures demonstrated that neither the polymer nor its degradation products are toxic.
|
|
The team produced polymer films that were equipped with functional side groups that act as “anchor points” for molecules, which can be used to attach fluorescence dyes and biomolecules. “Our new degradable polymer films could find broad application for the functionalization and coating of surfaces in the biological sciences as well as medicine and for food packaging applications,” states Lahann.
|

