| Posted: Feb 15, 2017 |
An old rock teaches us new tricks to fabricate van der Waals heterostructures
(Nanowerk News) In 2004 André Geim and Konstantin Novoselov proved that it is possible to separate atomically-thick layers of graphite and other two-dimensional materials by just using an adhesive tape. Since then, the scientific community has pursued the possibility of building man-made materials by artificial stacking of different ultrathin materials one on top of the other. These materials are called van der Waals heterostructures.
|
|
Until now, these heterostructures were completely handcrafted and the fabrication procedures presented several drawbacks such as the difficulty to align the crystal lattices (with atomic accuracy) of the different adjacent materials or to avoid trapping ambient adsorbates between the layers, hampering their performance and reproducibility.
|
 |
| Spanish playing cards used to represent the concept of heterostructures. (Left) Playing cards stacked in perfectly aligned layers, just as it happens in franckeite. (Right) Playing cards showing stacking defects as it happens in manmade van der Waals heterostructures. (Image: IMDEA)
|
|
Researchers from IMDEA Nanoscience (Madrid, Spain), Universidad Autónoma de Madrid (Spain), ALBA synchrotron and Delft University of Technology have realized that Nature is one step ahead and it has already solved these issues.
|
|
The researchers found out that franckeite, a mineral belonging to the sulfosalts family, shows a natural crystal structure similar to the manmade van der Waals heterostructures – with the huge advantage of an almost perfect alignment between crystal lattices and the absence of tapped residues between layers.
|
|
The researchers were amazed to see how Nature fabricates better van der Waals heterostructures than scientists do in their laboratory.
|
|
The researchers have shown that similar procedures as those employed in the isolation of graphene can be used to obtain few-atoms thick layers of franckeite allowing them to fabricate infrared photodetectors and solar cells.
|
|
These devices are highly interesting for several applications such as night-vision systems (like video cameras) or telecommunications sensors.
|
|
Furthermore, the researchers also demonstrated that franckeite can be exfoliated in liquid-phase, obtaining suspensions of the material in a solvent that ultimately leads to the fabrication of large-scale ultrathin layers.
|
|
These methods were not compatible with the handmade procedures used to fabricate heterostructures before, which only allowed to stack layer-by-layer one at a time.
|
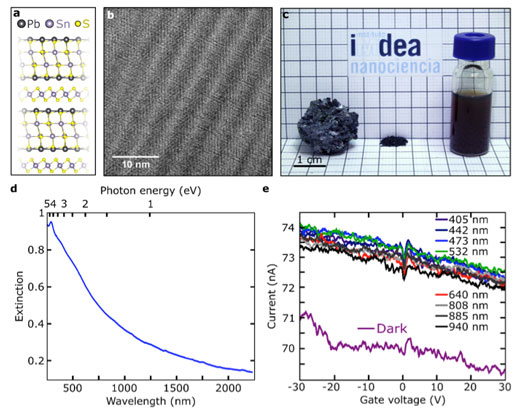 |
| a) Franckeite crystal structure. b) High-resolution transmission electron microscopy of a mechanically exfoliated franckeite flake. c) Franckeite samples. Left: bulk material; middle: powder material; right: suspension of exfoliated material prepared by sonication of a powder dispersion in NMP. d) UV-Vis-NIR spectrum in CDCl3 of the colloidal suspension shown in c-right. e) Current as a function of the applied back-gate voltage in a franckeite photodetector in dark conditions and upon ilumination with lasers of different wavelengths with the same power. (Image: IMDEA)
|
|
This work has been recently published in Nature Communications (("Franckeite as a naturally occurring van der Waals heterostructure") in open access format.
|
|
The isolation of franckeite as a few-atoms thick material becomes a milestone in the two-dimensional materials research field because, from now on, it is possible to obtain van der Waals heterostructures from nature itself, avoiding their complicated synthesis.
|


