| May 15, 2014 |
Learning from sharks how to stabilize genetically engineered antibodies
|
|
(Nanowerk News) Genetically engineered antibodies are deployed successfully in cancer diagnostics and therapy. Therapeutic antibodies against Alzheimer’s disease and multiple sclerosis are currently under development. An important criterion when designing suitable antibody fragments is their stability. Comparing the antibodies of sharks, which are very old from an evolutionary perspective, with those of humans, a team of researchers at the Technische Universitaet Muenchen (TUM) and the Helmholtz Zentrum Muenchen discovered stabilizing mechanisms that can also be applied to optimize custom-tailored antibodies in humans.
|
|
Custom-tailored antibodies are regarded as promising weapons against a multitude of serious illnesses. Since they can accurately recognize specific structures on the surface of viruses, bacteria or cancer cells, they are already being deployed successfully in cancer diagnostics and therapy, as well as against numerous other diseases. The stability of the sensitive antibodies is a decisive factor in every step, from production and storage to therapeutic application.
|
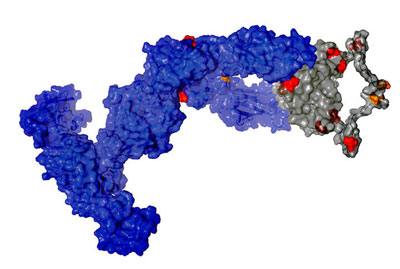 |
| Structural model of the IgNAR shark antibody. (Image: Janosch Hennig, TUM/Helmholtz Zentrum)
|
|
A team of researchers headed by Dr. Matthias J. Feige and the professors Linda Hendershot of St. Jude Children’s Research Hospital in Memphis (Tennessee, USA), Michael Sattler (Chair of Bimolecular NMR Spectroscopy at TU Muenchen and the Institute for Structural Biology at the Helmholtz Zentrum Muenchen), Michael Groll (Chair of Biochemistry at TU Muenchen) and Johannes Buchner (Chair of Biotechnology at TU Muenchen) set out to find new approaches for stabilizing antibodies by comparing mammalian antibodies with those of sharks.
|
|
Sharks have been in existence for over 500 million years. From an evolutionary perspective they are among the oldest animals with a “modern” immune system not unlike that of humans. Shark blood contains large quantities of urea, allowing the animal to survive in salt water. While urea protects sharks from dehydration, it can also destabilize sensitive protein molecules such as antibodies.
|
|
“Human antibodies would collapse under these conditions. Shark antibodies must, therefore, possess structural characteristics that make them particularly robust,” says Matthias J. Feige, lead author of the publication in PNAS ("The structural analysis of shark IgNAR antibodies reveals evolutionary principles of immunoglobulins"). “We wanted to unravel this mystery.”
|
|
Meticulous puzzle work
|
|
The researchers chose the IgNAR (immunoglobulin new antigen receptor) shark antibody for their investigations. Lacking structural information on the molecule and due to the fact that the molecule could not be completely crystallized, they developed a model of the antibody in detective-like puzzle work.
|
|
They crystallized parts of the antibody and determined their atomic structures using X-ray crystallography, comparing the segments with previously known structures of other immunoglobulins. For other antibody segments, they analyzed the structures using NMR spectroscopy. Time and again the researchers compared the determined structures and spatial distances with the results of X-ray diffraction measurements of natural shark antibody molecules to then finally construct a comprehensive model of the IgNAR antibody.
|
|
Precise examination of the protein’s structure revealed that the Ig folding typical for antibodies had already developed over 500 million years ago, since it is also present in sharks. Moreover, the researchers were able to identify the source of the of shark antibody stability: It results from an additional salt bridge between structurally important amino acid chains and a particularly large non-polar nucleus of the Ig fold in shark antibodies.
|
|
|
|
Human antibodies with enhanced stability
|
|
The researchers succeeded in integrating both stabilizing principles into human antibodies. In fact, the combination of both principles led to a significant increase of stability of the human antibody fragments. Their melting point was 10 degrees higher than that of the original molecule.
|
|
The increased stability also showed a positive effect in mammalian cells that typically produce therapeutic antibodies: The altered antibodies were produced in significantly larger quantities. In the near future, so the expectation of the researchers, this insight will enable the development of improved therapeutic and diagnostic antibodies. They should become easier to fabricate and store, as well as remain active longer inside the human organism, allowing them to unfurl their full therapeutic potential.
|

