Silver Nanoparticles (AgNPs): Properties, Synthesis, and Multifaceted Applications
What are Silver Nanoparticles?
Silver nanoparticles (AgNPs) are ultrafine particles of silver with dimensions ranging from 1 to 100 nanometers (nm). At this nanoscale size, silver exhibits unique physical, chemical, and biological properties that differ from bulk silver. These properties make AgNPs highly attractive for a wide range of applications, from antimicrobial agents and catalysts to sensors and biomedical devices.
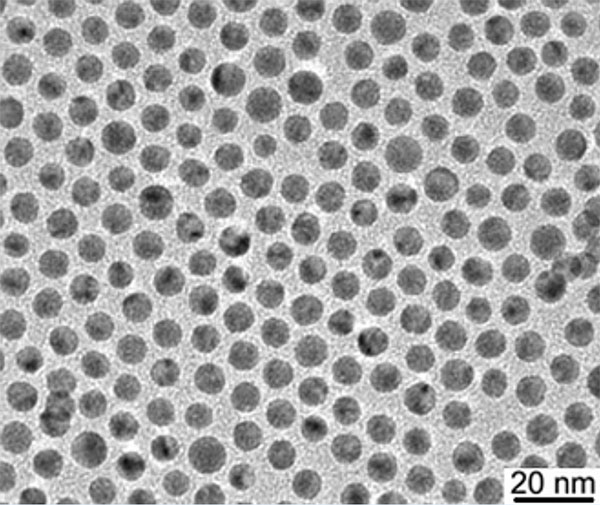
Synthesis Methods
Silver nanoparticles can be synthesized through various methods, including:
- Chemical Reduction: This is the most common method, involving the reduction of silver ions (Ag+) in a solution using reducing agents like sodium borohydride or citrate. The size and shape of the nanoparticles can be controlled by adjusting reaction conditions such as temperature, pH, and concentration of reactants.
- Physical Methods: AgNPs can also be produced by physical methods such as laser ablation, evaporation-condensation, and electrical discharge. These methods typically involve the generation of silver vapors, which then condense to form nanoparticles.
- Biological Synthesis: Green synthesis methods using biological entities like plants, fungi, and bacteria have gained attention due to their eco-friendliness and low toxicity. These organisms contain natural reducing agents that can convert silver ions into nanoparticles.
Properties and Characterization
Silver nanoparticles possess several unique properties that make them attractive for various applications:
- Optical Properties: AgNPs exhibit strong surface plasmon resonance (SPR) absorption in the visible region, which can be tuned by controlling their size and shape. This property makes them useful for optical sensing, imaging, and surface-enhanced Raman scattering (SERS).
- Antimicrobial Activity: AgNPs are known for their potent antimicrobial activity against a wide range of bacteria, fungi, and viruses. The mechanism of action involves the release of silver ions, which disrupt cell membranes, interfere with DNA replication, and generate reactive oxygen species.
- Catalytic Activity: AgNPs have been explored as catalysts for various chemical reactions, such as the reduction of nitroaromatic compounds and the oxidation of organic molecules. Their high surface-to-volume ratio and unique electronic properties contribute to their catalytic efficiency.
To characterize silver nanoparticles, several techniques are commonly used, including:
- Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM) for imaging and size analysis
- UV-Visible Spectroscopy for studying optical properties and SPR
- X-ray Diffraction (XRD) for determining crystal structure and purity
- Dynamic Light Scattering (DLS) for measuring hydrodynamic size and size distribution
- Zeta Potential for assessing surface charge and stability
Applications
Silver nanoparticles find applications in diverse fields due to their unique properties:
Antimicrobial Applications
AgNPs are incorporated into a wide range of products for their antimicrobial properties, such as wound dressings, medical devices, water purification systems, textiles, and food packaging materials. They offer a potential alternative to conventional antibiotics in combating the rise of antibiotic-resistant bacteria.
Biomedical Applications
AgNPs are being explored for various biomedical applications, including targeted drug delivery, bioimaging, biosensing, and cancer therapy. Their unique optical properties and biocompatibility make them promising candidates for theranostic applications, combining both diagnostic and therapeutic functions.
Catalytic Applications
AgNPs are used as catalysts in chemical reactions such as the reduction of nitroaromatic compounds, which has implications in environmental remediation. They are also employed in the synthesis of fine chemicals and pharmaceuticals, offering high selectivity and efficiency.
Sensing and Detection
The optical properties of AgNPs are harnessed for the development of sensitive and selective sensors for various analytes, including biomolecules, metal ions, and small organic molecules. AgNP-based sensors find applications in environmental monitoring, food safety, and clinical diagnostics.
Safety and Environmental Considerations
While silver nanoparticles offer numerous benefits, there are also concerns regarding their potential toxicity and environmental impact. AgNPs can interact with biological systems and cause oxidative stress, DNA damage, and cellular toxicity. The release of AgNPs into the environment may also have ecological consequences, affecting aquatic organisms and soil microbial communities.
To address these concerns, ongoing research focuses on understanding the mechanisms of AgNP toxicity, developing safer synthesis methods, and implementing proper disposal and recycling strategies. Regulatory bodies are also working on establishing guidelines for the safe use and handling of AgNPs in various applications.
Future Perspectives
As research on silver nanoparticles continues to advance, new applications and opportunities are expected to emerge. Some future directions include:
- Development of multifunctional AgNPs with combined antimicrobial, catalytic, and sensing properties
- Exploration of AgNP-based nanocomposites and hybrid materials for enhanced performance
- Integration of AgNPs with other nanomaterials for synergistic effects
- Advancement of green synthesis methods using renewable resources and mild reaction conditions
- Elucidation of the long-term fate and impact of AgNPs in biological and environmental systems
With continued research and responsible development, silver nanoparticles hold great promise for addressing various challenges in healthcare, environmental protection, and technological innovation.
Further Reading
International Journal of Molecular Sciences, Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches
International Journal of Molecular Sciences, Silver Nanoparticles and Their Antibacterial Applications
Ecotoxicology and Environmental Safety, Synthesis, applications, toxicity and toxicity mechanisms of silver nanoparticles: A review
