| Posted: Oct 18, 2017 |
Researchers customize catalysts to boost product yields, decrease separation costs
(Nanowerk News) For some crystalline catalysts, what you see on the surface is not always what you get in the bulk, according to two studies led by the Department of Energy's Oak Ridge National Laboratory.
|
|
The investigators discovered that treating a complex oxide crystal with either heat or chemicals caused different atoms to segregate on the surface, i.e., surface reconstruction. Those differences created catalysts with dissimilar behaviors, which encouraged different reaction pathways and ultimately yielded distinct products.
|
|
By using thermal and chemical treatments, catalyst designers may be able to drive industrially important chemical reactions to improve yields of desired products and reduce unwanted products so post-reaction separation costs can be significantly lowered.
|
|
"The surface of a catalyst is a playground for the molecules to do the chemical reaction," said ORNL chemist Zili Wu, the senior author of two recent papers about the effect of the atomic composition of a catalyst surface on acid-base chemistry. "If you can tune your catalyst to obtain the desired product, i.e., achieve high selectivity, you will reduce the side products. Then you don't need costly and energy-intensive downstream chemical separation as much."
|
|
The researchers surveyed four catalysts of perovskite, a mixed oxide crystal made of cubic unit cells of the atomic composition ABO3, with A as a rare-earth metal cation (positively charged ion), B as a transition-metal cation and O as oxygen.
|
|
Treating a perovskite with heat resulted in a catalyst with more A atoms on its surface, scientists including first co-authors Guo Shiou Foo and Felipe Polo-Garzon reported in ACS Catalysis ("Acid–Base Reactivity of Perovskite Catalysts Probed via Conversion of 2-Propanol over Titanates and Zirconates").
|
|
Treating the same perovskite with chemicals instead produced more B atoms on the surface, scientists including first author Polo-Garzon subsequently reported in Angewandte Chemie International Edition ("Controlling Reaction Selectivity through the Surface Termination of Perovskite Catalysts").
|
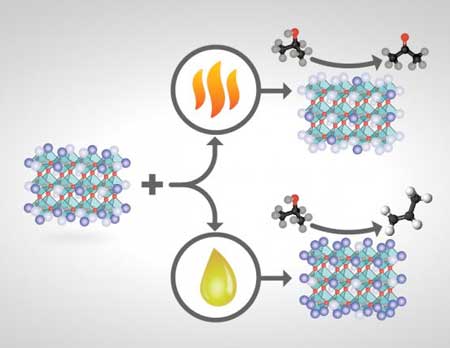 |
| How perovskite catalysts are made and treated changes their surface compositions and ultimate product yields. If certain perovskite catalysts of the formula ABO3 are heat-treated, the catalyst's surface terminates predominantly with A (a rare-earth metal cation depicted in light purple) and less with B (a transition-metal cation shown in dark purple)--and isopropanol conversion over this basic catalyst primarily yields acetone. If the same catalyst is treated chemically instead of with heat, the catalyst's surface termination is instead mostly B and less A and is more acidic--and isopropanol conversion yields mainly propylene. (Image: Oak Ridge National Laboratory, U.S. Dept. of Energy; illustrator Adam Malin)
|
|
The scientists were the first to systemically study how different perovskite surface compositions affect acid-base catalysis. The knowledge gained could provide a route to selective conversion of biomass into value-added chemicals.
|
|
To test the acid-base performance of the treated perovskite catalysts, the researchers studied a model reaction, the conversion of isopropanol--basically, rubbing alcohol. Depending on the pre-treatment conditions, the perovskite could selectively turn the alcohol into propylene, a building block of plastics, through a dehydration reaction, or acetone, an industrial solvent, through a dehydrogenation reaction.
|
|
"Isopropanol adapts to your catalyst's surface," Wu explained. "If you have a basic surface (an AOx-dominated surface), it will do the base-catalyzed reaction (to acetone). If you have an acid surface (a BOx-dominated surface), it adapts to that route (to propylene). So isopropanol is a good probe molecule to tell you the surface composition of the catalyst."
|
|
The experiments showed a wide range of tunability was possible with different treatments. The same perovskite starting material, subjected to different treatments, could yield a desired product, such as acetone or propylene, in a wide range, from 25 to 90 percent.
|
|
In experiments Wu conceived, Foo and Polo-Garzon used X-ray diffraction to characterize the bulk of a catalyst and numerous techniques to characterize its surface. To learn if element A or B predominated on the perovskite surface if the catalyst was subjected to heat or chemical pretreatments, Shi-Ze Yang, supervised by Matthew Chisholm, did scanning transmission electron microscopy of catalyst nanoparticles, whereas Foo used adsorption microcalorimetry and infrared spectroscopy.
|
|
Low-energy ion scattering, performed at Lehigh University, shot an ion at a nanoparticle, and the energy lost when the ion bounced back revealed compositional details of the very top surface layer, which is critical for catalysis. Lessons learned about surface composition from all these experiments aided Victor Fung and De-en Jiang in theory-based computations to predict reaction pathways.
|
|
Polo-Garzon and Elizabeth Bickel, a summer student from Tennessee Technology University, conducted measurements that confirmed the impact of surface segregation on the acid-base catalytic properties of the perovskite material.
|
|
What's next? The researchers would like to further explore reconstruction processes of perovskite catalyst surfaces with different termination facets. "The geometry and the composition of the cation and anion [negatively charged ion] are arranged differently when you have different facets," Wu explained. "That can give you quite a different chemical reactivity."
|
|
Also, the researchers are currently expanding their work to tune the surface terminations of perovskites to understand and optimize oxidation and reduction reactions beyond acid-base ones, which could be used in the conversion of shale gas (mostly methane) to valuable chemicals.
|

