| Jan 15, 2020 | |
The mysterious movement of water molecules(Nanowerk News) Water is ubiquitous and essential for life. Nevertheless, experimental information about its behaviour on the atomic level – above all how it interacts with surfaces – is scarce. Thanks to a new experimental method, TU Graz researchers have now delivered insights into the atomic-level movement of water molecules, which they outline in a paper in Nature Communications ("Nanoscopic diffusion of water on a topological insulator"). |
|
 |
|
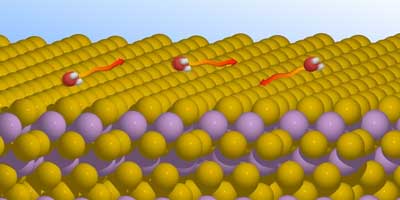
| Schematic representation of the movement of water molecules on a topological insulator. (Image: Tamtögl) | |
| Water is a mysterious substance. Understanding its behaviour on an atomic scale remains a challenge for experimentalists as the light hydrogen and oxygen atoms are difficult to observe with conventional experimental probes. This is especially true when trying to observe the microscopic movement of individual water molecules which occurs on a pico-second time-scale. | |
| As reported in their paper, researchers from the Exotic Surfaces group at TU Graz’s Institute of Experimental Physics joined forces with counterparts from the Cavendish Laboratory at the University of Cambridge, the University of Surrey and Aarhus University. | |
| Together, they made significant advances, performing research into the behaviour of water on a material which is currently attracting particular interest: the topological insulator bismuth telluride. | |
| This compound could be used to build quantum computers. Water vapour would then be one of the environmental factors to which applications based on bismuth telluride may be exposed during operation. | |
| In the course of their research, the team used a combination of a new experimental method called helium spin-echo spectroscopy and theoretical calculations. Helium spin-echo spectroscopy utilises very low-energy helium atoms which allows the motion of isolated water molecules to be observed without disruption. | |
| The researchers discovered that water molecules behave completely different on bismuth telluride compared to those on conventional metals. They report clear evidence for repulsive interactions between water molecules, which is contrary to the expectation that attractive interactions dominate the behaviour and aggregation of water on surfaces. | |
| Bismuth telluride appears to be insensitive to water, which is an advantage for applications under typical environmental conditions. Plans are in place for further experiments on similarly structured surfaces, intended to clarify whether the movement of water molecules is attributable to specific features of the surface in question. |
| Source: Technische Universität Graz | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
