| Jul 31, 2020 | |
Unusual electron sharing found in cool crystal(Nanowerk News) A team of scientists led by Nagoya University in Japan has detected a highly unusual atomic configuration in a tungsten-based material. Until now, the atomic configuration had only been seen in trihydrogen, an ion that exists in between star systems in space. |
|
| The findings, published in the journal Nature Communications ("Regular-triangle trimer and charge order preserving the Anderson condition in the pyrochlore structure of CsW2O6"), suggest further studies could reveal compounds with interesting electronic properties. | |
 |
|
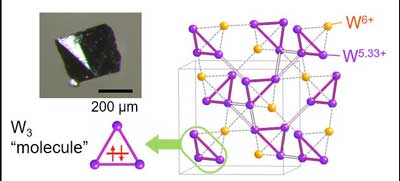
| When CsW2O6 is cooled below -58 °C, molecular triangles form of tungsten atoms that are bonded together by only two electrons. Similar bonding has only previously been demonstrated in trihydrogen ions in outer space. (Image: Yoshihiko Okamoto) | |
| Atoms that make up humans and trees and kitchen tables generally bond together by sharing electrons - think of electrons as the atomic glue of life. Nagoya University applied physicist Yoshihiko Okamoto and colleagues have found a highly unusual version of this glue: a regular triangular molecule was formed of three atoms bonded together by two electrons. | |
| "This type of bond had only previously been seen in the trihydrogen ions found in interstellar material," says Okamoto. "We were excited to see this configuration in a cooled tungsten-based crystal." | |
| The so-called tritungsten molecules were discovered in single crystals of caesium tungsten oxide (CsW2O6) cooled below -58 °C. CsW2O6 conducts electricity at room temperature but changes into an insulating material when it is cooled below -58 °C. | |
| It has been a challenge to study how the atomic structure of this type of material changes in response to temperature. To overcome this, Okamoto and his colleagues in Japan synthesized very pure single crystals of CsW2O6 and bombarded them with X-ray beams at room temperature and -58 °C. | |
| The tungsten molecules in the conducting crystal form three-dimensional networks of tetrahedral pyramids connected at their corners, known as a pyrochlore structure. The bonds between the molecules form due to a symmetrical sharing of electrons between them. | |
| However, when the compound is cooled, the electrons re-arrange and two types of tungsten atoms emerge within the tetrahedra, each with a different 'valence', or bonding power with other atoms. This, in turn, distorts the lengths of tungsten bonds with oxygen atoms in the compound, leading to a more compressed shape. | |
| Importantly, the tungsten atoms with lower valence form small and large triangles on the sides of the tungsten tetrahedra, with the highly unusual tritungsten molecules forming on the small triangles. The three tungsten atoms forming the points of these triangles share only two electrons between them to keep them bonded together. | |
| "To our knowledge, CsW2O6 is the only example where this type of bond formation, where several atoms share only a few electrons, appears as a phase transition," says Okamoto. | |
| The team aims to further investigate compounds with pyrochlore structures, with the ultimate goal of discovering materials with new and interesting properties. |
| Source: Nagoya University | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
