| Aug 24, 2020 | |
Researchers develop ultra-long circulating nanoparticle to treat chronic myeloid leukemia(Nanowerk News) By conjugating CHMFL-ABL-053 to an amphiphilic polymer and self-assembling into a nanoparticle (NP) with a high loading, an ultra-long circulating nanomaterial was prepared by researchers recently (Nanomedicine: Nanotechnology, Biology and Medicine, "An ultra-long circulating nanoparticle for reviving a highly selective BCR-ABL inhibitor in long-term effective and safe treatment of chronic myeloid leukemia"). |
|
| Developed by Drs. FU Liyi and ZOU Fengming led by Profs. LIU Qingsong and LIU Jing from the Institute of Health & Medical Technology, Hefei Institutes of Physical Science, the formulation could greatly improve its solubility and drastically extended its circulation half-life. | |
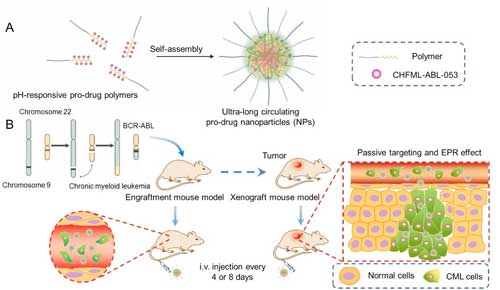 |
|
| (A) Self-assembly of the NPs and (B) their targeting of CML on two models. (Image courtesy of the research team) (click on image to enlarge) | |
| Chronic myeloid leukemia (CML) is a clonal hematopoietic stem cell myeloproliferative disease, which is primarily caused by the chromosomal translocation between the Abelson (ABL) gene and the breakpoint cluster region (BCR) gene. Although the FDA-approved BCR-ABL inhibitors, such as Imatinib and Dasatinib etc. could greatly improve the 10-year survival rate of the patients, their off-targets, such as DDR1/2 and c-kit, may lead to the decrease of mast cells, vascular adverse events and other undesired side effects. | |
| Previously related research was conducted by Prof. LIU Qingsong and Prof. LIU Jing's group. And the BCR-ABL inhibitor CHMFL-ABL-053 had a better selectivity to the target of BCR-ABL over other protein kinases. However, like all of this class of inhibitors, it must be treated orally every day due to its short half-life, which would not only increase the economic burden of patients, but also lead to cumulative toxicities. | |
| This time, the team pushed their work further to make a modification based on their previous work. In the 150 days' long-term engraftment model experiment, long intravenous dosing intervals of the NPs (every 4 or 8 days) exhibited much better survival and negligible toxicities as compared to daily oral administration of the inhibitor. | |
| The NPs showed excellent inhibition of tumor growth in the subcutaneous xenograft model. The excellent anti-leukemic efficacy of the NPs in the long injection cycle on both models might provide a novel, effective and safe therapeutic strategy for BCR-ABL-positive CML. |
| Source: Chinese Academy of Sciences | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
