| Oct 22, 2020 | |
Oxygen can do a favor to synthesize metal-organic frameworks(Nanowerk News) Metal-organic frameworks, or MOFs, are composed of metal ions periodically surrounded by organic bridging molecules, and these hybrid crystalline frameworks feature a cage-like hollow structure. |
|
| This unique structure motif offers great potential for a range of applications in energy storage, chemical transformations, optoelectronics, chemiresistive sensing, and (photo)electrocatalysis, among others. | |
| Debuted in the early 2000s, MOFs are a fascinating nanomaterial. Though numerous applications exploit MOFs, little has been known as to how oxygen may work in the synthesis of MOFs. | |
| Led by Director Rodney S. Ruoff and senior chemist Dr. Yi Jiang, chemists from the Center for Multidimensional Carbon Materials (CMCM) within the Institute for Basic Science (IBS) located at Ulsan National Institute of Science and Technology (UNIST) in collaboration with their colleagues at UNIST and Sungkyunkwan University (SKKU) have identified how oxygen affects the synthesis of a novel MOF; copper 1,3,5-triamino-2,4,6-benznetriol metal-organic framework [Cu3(TABTO)2-MOF]. | |
| Their findings were published in a recent article in the Journal of the American Chemical Society ("Synthesis of a Copper 1,3,5-Triamino-2,4,6-benzenetriol Metal–Organic Framework"). | |
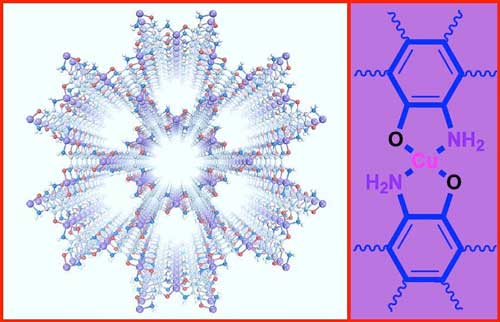 |
|
| Figure 1.The structure of the Cu3(TABTO)2-MOF (carbon, nitrogen, oxygen, hydrogen, and copper atoms are gray, blue, red, white, and purple, respectively). (Image: IBS) (click on image to enlarge) | |
| “Since organic redox-active ligands are usually sensitive to oxygen, the presence of oxygen is not favored in many organic reactions. However, oxygen can be helpful for the synthesis of some redox-active ligand-based MOFs, but many chemists did not realize this,” notes Dr. Yi Jiang, the first author of the study. The researchers synthesized a 2D conjugated MX2Y2-type (M = metal, X, Y = N, S, O, and X != Y) Cu3(TABTO)2-MOF based on a redox-active ligand (1,3,5-triamino-2,4,6-benzenetriol). | |
| The role of oxygen in the synthesis of this MOF was identified by comparing the results from experiments in air and inert gas (argon): Pure Cu3(TABTO)2-MOF was produced in the presence of oxygen, but the Cu3(TABTO)2-MOF together with copper metal was formed if oxygen was absent. | |
| Dr. Jiang adds, “Our study suggests that oxygen prevents these ligands from reducing the Cu (I and II) ions to Cu metal, facilitating the synthesis of a pure MOF.” | |
| They also revealed that Cu3(TABTO)2-MOF became electrically conductive after being chemically oxidized by iodine because of the formation of CuI and carriers. It is originally an insulator with almost no electrical conductivity. | |
| The iodine-doping generates 0.78 siemens per centimeter of electrical conductivity in the Cu3(TABTO)2-MOF pellet that was synthesized in air. Further experiments and analysis found the metallic characteristics of the materials. | |
| Modelling the structure via detailed density functional theory (DFT) calculations, the researchers also experimentally studied the structure of this 2D MOF through X-ray diffraction, diffuse reflectance UV-vis, X-ray photoelectron, electron paramagnetic resonance, and Raman spectroscopies. | |
| “Our work contributed to a fundamental understanding of the role of oxygen in the synthesis of redox-active ligands-based MOFs, and should inspire the community to pay more attention to the role oxygen can play in synthesis of redox-active ligands-based MOFs,” says Director Rodney S. Ruoff, the corresponding author of the study. Dr. Jiang further explains, “Most work in this field focused on the synthesis of MX4-type (M = metal, X = N, O, or S) MOFs based on redox-active ligands. The synthesis of new electrically conductive MOFs that are not the MX4-type is both challenging and meaningful work. Both the as-synthesized and iodine-doped Cu3(TABTO)2-MOFs might be useful in catalysis and energy-related applications.” | |
| UNIST and SKKU collaborators measured physical properties and did theoretical modeling of this new MOF. Prof. YOO Jung-Woo and his student OH Inseon of the Department of Materials Science and Engineering at UNIST studied the iodine doping-induced electrical conductivity of the Cu3(TABTO)2-MOF. | |
| The metallic characteristics of this iodine-doped material were also explored by Prof. HWANG Jungseek and his Postdoctoral Fellow Dr. SEO Yu-Seong of the Department of Physics at Sungkyunkwan University. Prof. KWAK Sang Kyu and his student JOO Se Hun of the School of Energy and Chemical Engineering at UNIST modeled the structure through detailed DFT calculations. |
| Source: Institute for Basic Science | |
| Share this: | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
|
