| Nov 09, 2020 |
3D model shows bacterial motor in action
(Nanowerk News) Nagoya University scientists in Japan and colleagues at Yale University in the US have uncovered details of how the bacterial propeller, known as the flagellum, switches between counterclockwise and clockwise rotation, allowing it to control its movement.
|
|
The findings were published in the journal eLife ("The flagellar motor of Vibrio alginolyticus undergoes major structural remodeling during rotational switching") and include a model that shows structural changes happening within portions of the flagellar motor.
|
|
Vibrio bacteria are rod-shaped organisms that live in coastal waters. They can cause serious intestinal and soft tissue infections that can ultimately lead to septic shock and multiple organ failure.
|
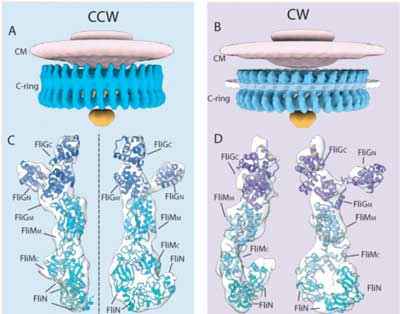 |
| The flagellar motor is formed of a series of rings bound by proteins. The scientists looked specifically at the C-ring (blue), which is present inside the bacterial cytoplasm. The C-ring is formed of FliG, FliN and FliM proteins. The scientists found that during the switch from counterclockwise to clockwise rotation, part of FliG moves, exposing its charged residues so they interact with the stator. (Image: Jun Liu) (click on image to enlarge)
|
|
"Vibrio infections are expected to increase as water temperatures rise due to climate change," says Nagoya University supramolecular biologist Michio Homma. "They have evolved a sophisticated flagellum-driven motility to facilitate their invasion of host organisms. We wanted to visualize how their motors switch between clockwise and counterclockwise rotation to further understand this movement."
|
|
To do this, Homma and his colleagues used an advanced imaging technique called cryo-electron tomography, in which images are taken of frozen samples as they are tilted to produce 2D images that are combined to produce a 3D reconstruction.
|
|
The scientists used samples from two mutant Vibrio bacteria whose flagella only rotated in the clockwise or counterclockwise direction. This allowed them to compare the two movements and deduce the changes happening within the bacteria's motor to switch directions.
|
|
"Our comparative analysis and molecular modelling provide the first structural evidence that the flagellar motor undergoes a profound rearrangement to enable the rotational switch," says Homma.
|
|
The scientists found that the switch from counterclockwise to clockwise involves a signalling protein, called CheY-P, binding to a protein, called FliM, in the flagellar motor's C-ring. This causes another motor protein, called FliG, to move in a way that exposes charged residues on its surface to a transmembrane protein, called PomA, that forms the stationary part of the motor, called the stator, along with another protein called PomB.
|
|
The interaction between FliG residues and PomA probably leads to changes in the stator that result in an ion flow generating torque, which ultimately rotates the C-ring.
|
|
"Cryo-electron tomography is rapidly evolving, making it increasingly possible to reveal motor structure at higher resolutions," says Homma. "This current study provides one of the highest resolution images by cryo-electron tomography of the Vibrio flagellar motor. This and future studies will further our understandings of the flagellar assembly and function."
|

