| Jul 13, 2021 |
Microwave-induced cancer treatment
(Nanowerk News) An international team led by physics researchers at The University of Texas at Arlington has published a paper in the high-impact journal Bioactive Materials ("Aggregation-induced emission luminogens for highly effective microwave dynamic therapy") that describes a breakthrough method of photodynamic therapy (PDT), an emerging cancer treatment.
|
|
Nil Kanatha Pandey, a doctoral student in Physics Professor Wei Chen’s lab, is the first author of the paper titled “Aggregation-induced emission luminogens for highly effective microwave dynamic therapy.” The study was managed by Chen in collaboration with Lingyun Wang, a professor of chemistry and chemical engineering at South China University of Technology.
|
|
PDT uses photosensitive molecules with light at the site of a tumor to produce powerful reactive oxygen species that destroy cancer cells. Researchers view PDT as a promising cancer treatment due to its minimal invasiveness and low side effects, but the process is not without obstacles.
|
|
“In human tissue, the depth that light can reach is limited,” Chen said. “For cancers located deep within an organ or muscle, PDT is less effective because we can’t deliver light to the site of the tumor.”
|
|
Conventional photosensitizers used in PDT are also dependent on oxygen, which is often absent near the site of tumors. As a result, the efficacy of PDT is significantly reduced in the context of the hypoxic environment.
|
|
But an alternate method of tumor destruction known as thermal ablation offers a pathway to overcoming the challenges of traditional PDT.
|
|
Thermal ablation is considered one of the most effective treatments in combined cancer therapy because it leads to improved tumor sensitivity to PDT, chemotherapy, immunotherapy and radiotherapy. During thermal ablation, the tissues are heated with microwave technology, causing the blood vessels to dilate, which increases blood flow. As hemoglobin in blood contains oxygen, heat boosts the amount of oxygen, thereby enhancing the efficacy of the treatment.
|
|
In its study, the team employed microwave technology to activate a special type of photosensitive molecule known as aggregation-induced emission luminogens (AIEgens). Upon investigation, Chen and his researchers discovered that heating the cancer-affected area with microwave irradiation and AIEgens allowed for deep tissue penetration and generated reactive oxygen species that killed the cancer cells.
|
|
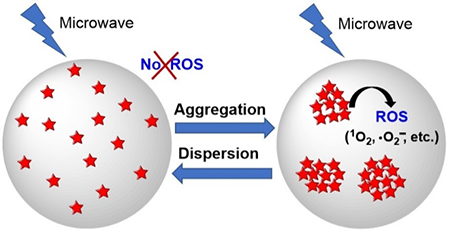
AIEgens are new kinds of materials that produce a phenomenon opposite to traditional photosensitizers. Aggregations at high concentrations quench the emission and reactive oxygen species (ROS) production for traditional photosensitizers. However, for AIEgens, instead of quenching, aggregations at high concentrations enhance the emission and ROS production, which benefits image-guided PDT.
|
 |
| Figure 1: Schematic illustration of AIEgen-mediated microwave-induced PDT. (Image: UTA)
|
|
“The microwave-induced PDT is a new phenomenon that has many advantages over traditional PDT,” Chen said. “Compared with other thermal therapies, microwave technology has many advantages, such as maneuverability, faster ablation time, and negligible side effects. Most notably, it allows for deeper penetration of tissue.”
|
|
Chen’s team believes that its findings not only solve the issues of traditional PDT but also help to improve conventional microwave ablation therapy by reducing the microwave dose needed to achieve the same outcomes, thereby decreasing the occurrence of side effects of microwave irradiation.
|
|
“The combination of AIEgens and microwave technology will lead to the discovery of more effective methods of cancer treatment for hard-to-reach tumors,” Wang said. “This finding will benefit investigations into PDT by researchers around the world. Our primary goal is to make PDT as effective and accessible for cancer patients as possible.”
|
|
“These nanoaggregates are very effective for producing ROS and killing cancer cells even at low concentration of the nanoaggregates and low power of the microwave, which will potentially reduce the toxicity to normal cells,” Pandey said.
|
|
These AIEgens can produce ROS only when compact nanoaggregates are formed.
|
![Study of intracellular ROS detection using DCFH-DA staining dye]() |
| Figure 2. Study of intracellular ROS detection using DCFH-DA staining dye. The increase in green fluorescence intensity shows ROS production. (Image: UTA) (click on image to enlarge)
|
|
The researchers conducted several studies to confirm that these nanoaggregates can produce ROS. Figure 2 demonstrates that when these nanoaggregates are stimulated by microwave, significant amounts of ROS can be produced when compared to their corresponding control.
|
|
The researchers also carried out MTT and live/dead assays to demonstrate microwave-induced toxicity. The results of MTT show that the combination of these AIEgens and MW radiation can effectively destroy cancer cells with average IC-50 values of 2.73 and 3.22 µM, respectively.
|
![Effect of TPEPy-I and TPEPY-PF6 nanoaggregates under 10 W of MW exposure]() |
| Figure 3. Effect of TPEPy-I and TPEPY-PF6 nanoaggregates under 10 W of MW exposure. Green fluorescence shows live cells, whereas red fluorescence represents dead cells. (Image: UTA) (click on image to enlarge)
|
|
The results of the live/dead cell viability assay are depicted in Figure 3, which demonstrates that the nanoaggregates combined with microwaves are very toxic to cancer cells.
|



