| Jul 31, 2021 |
Watching light break down a model photocatalyst in near real time
(Nanowerk News) Chemists create catalysts for use in industry and other applications. One of the methods to create these catalysts is by using light to break down organometallic compounds—substances that include both metals and carbon. These types of compounds are called photocatalysts. Scientists call the process of breaking down molecules with light, photodissociation.
|
|
Researchers often study the photodissociation of iron pentacarbonyl as a model for understanding other catalysts. This study used a method called ultrafast infrared (IR) spectroscopy to study how ultraviolet light photodissociates gas phase iron pentacarbonyl.
|
|
Researchers know a great deal about the photochemistry of iron pentacarbonyl in the solution phase. However, scientists need combined experimental and theoretical gas phase studies to investigate the role of the molecule’s complex electronic structure on its photodissociation processes, which can help scientists better understand how the effects of solvents change reaction dynamics.
|
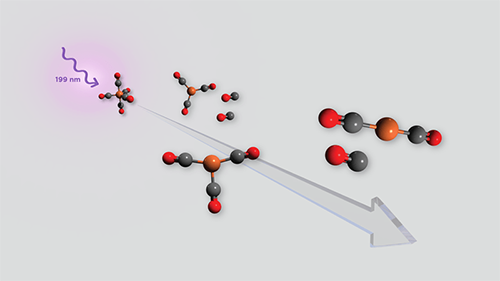 |
| Depiction of the proposed mechanism for the breakdown of iron pentacarbonyl when exposed to ultraviolet light at 199 nanometer wavelength. (Image: Research Publishing International)
|
|
This research (Journal of Chemical Physics, "Ultraviolet Photodissociation of Gas-Phase Iron Pentacarbonyl Probed with Ultrafast Infrared Spectroscopy") provides important new insights on the mechanisms, energetics, and timescales of the photodissociation of gas phase iron pentacarbonyl.
|
|
These fundamental science insights may help scientists design new organometallic photocatalysts for industry and other applications.
|
|
Iron pentacarbonyl [Fe(CO)5] interacts with ultraviolet (UV) light to produce reactive catalytic species that activate certain chemical bonds.
|
|
In this study, researchers investigated the mechanisms of UV-induced breakdown of iron pentacarbonyl in the gas phase using ultrafast IR spectroscopy combined with high-level quantum chemical calculations. They exposed gas phase iron pentacarbonyl to UV light in a 265 nanometer or 199 nanometer pulse, and then performed transient IR spectroscopy. This use of ultrafast IR spectroscopy enabled rapid chemical changes to be measured in real time.
|
|
Irradiating iron pentacarbonyl at 265 nm produces a short-lived intermediate, iron tetracarbonyl [Fe(CO)4] in a singlet excited state. This research identified this intermediate, providing evidence for the previously postulated sequential dissociation mechanism. The loss of another carbonyl (CO group) leads to the formation of iron tricarbonyl [Fe(CO)3] in a singlet excited state on a 3.4 picosecond timescale.
|
|
Then, over approximately 10 picoseconds, the research found evidence of the redistribution of energy or structural evolution of Fe(CO)3. Studies of 199 nanometer irradiation suggest production of singlet-excited Fe(CO)3 in less than 0.3 picoseconds, followed by intersystem crossing to the ground triplet states of Fe(CO)3 or iron dicarbonyl [Fe(CO)2] on a 15 picosecond timescale.
|
|
These results indicate carbonyl elimination mechanisms involving electronically and vibrationally excited species.
|

