| Nov 04, 2021 |
The intriguing behaviour of magnets with an extra bit of oxygen
(Nanowerk News) Below -148 °C (125 Kelvin), magnetite (Fe3O4) – the oldest magnetic material known to us – changes from metal to insulator. At that particular temperature, the material undergoes drastic transformations in its crystal structure, as well as its electric and thermal properties. Known as the Verwey transition, this physical process was discovered over 80 years ago and remains arguably one of the most important and challenging problems in condensed matter physics, still lacking a complete understanding.
|
|
Using magnetite nanoparticles, researchers at Seoul National University and colleagues explored the effects of the introduction of oxygen (oxygen doping) on magnetite. They oxidised magnetite nanoparticles at room temperature and checked the changes in their Verwey transition temperature over a period of three years.
|
|
Oxidation changes the charge of the iron in the magnetite and affects the ordering pattern inside the material. As more and more oxygen molecules penetrate deep into the magnetite nanoparticles, the Verwey transition temperature should gradually fall to a minimum value.
|
|
Instead, the researchers observed an intriguing and previously unreported variation of the Verwey transition temperature: it lowered to a minimum of approximately -203 °C (70 Kelvin) after 72 days of oxidation before rising to -178 °C (95 Kelvin). The team has explained this phenomenon with an elegant diffusion model.
|
|
The researchers calculated how many oxygen atoms could diffuse into the nanoparticles over time. They chose to use nanoparticles because studying the Verwey transition upon oxidation at room temperature in a larger block of magnetite would be prohibitively slow.
|
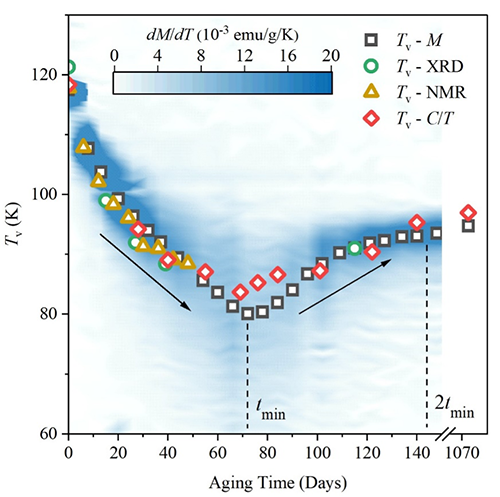 |
| While the Verwey transition happens at around -148 °C (125 Kelvin) in bulk magnetite, the analysis of nanoparticles of magnetite doped with oxygen at room temperature shows the drop and recovery of the Verwey transition temperature. The researchers followed this process for three years. (Image: SNU)
|
|
“Nanoparticles have a large surface where oxidation can take place, so they allow us to study this process on a reasonable timescale. The same experiment on a larger piece of magnetite would require an incredible number of years… I would say it would take forever,” explains Taehun Kim, one of the researchers who took part in this study (Nature Communications, "Slow oxidation of magnetite nanoparticles elucidates the limits of the Verwey transition").
|
|
It is still a slow experiment though. After approximately 140 days, only about 70 oxygen atoms are incorporated into each magnetite nanoparticle per day. The team took advantage of this slow pace to understand various aspects and limits of the oxidation process: while at the beginning of the oxidation process oxygen concentration is higher in the outer than inner layers of the nanoparticles, this initial gradient reduces over time.
|
|
“We suggested that the increase and decrease of the oxygen concentration gradient may be related to the drop and recovery of the Verwey transition temperature,” points out Kim. “However, it is unclear why such a small quantity of oxidation (or doping) has such a big influence on the Verwey transition. This could be the next direction of this research.”
|
|
“A much bigger lesson we learned from this work is that our experimental approach can be adapted to many different nanoparticles. This will open a completely new door to the oxidation problem on nanometer scales. If successful, it will have far-reaching implications that go beyond advances in our understanding of the effect of oxidation on the Verwey transition in magnetite," says Je-Geun Park, who led this study at Seoul National University.
|

