| Feb 15, 2022 |
Development of metastable-phase advanced material synthesis technology
(Nanowerk News) Similar to the widespread interest in graphite and diamond, there is growing interest in metastable phases, which have different physical properties than those of stable phases. However, processes to fabricate metastable-phase materials are highly limited.
|
|
Novel findings have been published about the development of a new metastable-phase synthesis method, which can drastically improve the physical properties of various materials.
|
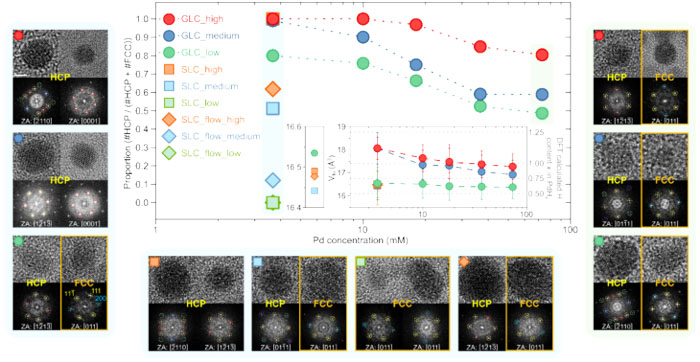 |
| The percentage of metastable-phase palladium hydrides (HCP) generated depended on the palladium concentration in the palladium aqueous solution and the electron beam intensity and content of hydrogen within the metastable phase. The percentage of metastable-phase palladium hydrides (HCP) generated depended on the palladium concentration in the palladium aqueous solution and the electron beam intensity and content of hydrogen within the metastable phase. (Image: Korea Institute of Science and Technology)
|
|
A research team led by Dr. Chun, Dong Won at the Clean Energy Research Division, Korea Institute of Science and Technology (KIST), announced that they successfully developed a new advanced metastable-phase palladium hydride (PdHx) material.
|
|
Furthermore, they identified its growth mechanism and published it in the latest issue of Nature ("Metastable hexagonal close-packed palladium hydride in liquid cell TEM").
|
|
A metastable-phase material has more thermodynamic energy than that in the stable phase but requires substantial energy to attain the stable phase, unlike most other materials, which exist in the stable phase with low thermodynamic energy.
|
|
The research team directly synthesized a metal hydride by growing a material that can store hydrogen under a suitable hydrogen atmosphere, without dispersing hydrogen within a metal.
|
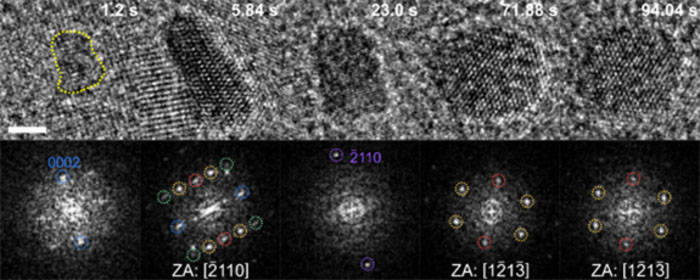 |
| Real-time analysis of the growth process of metastable palladium hydride nanoparticles within a liquid phase by transmission electron microscopy. (Image: Korea Institute of Science and Technology)
|
|
Notably, they successfully developed a metastable-phase metal hydride with a new crystal structure. Further, they confirmed that the developed metastable-phase material had good thermal stability and twice the hydrogen storage capacity of a stable-phase material.
|
|
To elucidate the theoretical basis and scientific evidence for these findings, the research team used atomic electron tomography, which reconstitutes 3D images from 2D electron microscope images for nanometer-sized crystals in a metal hydrate, for analysis. |
|
As a result, they demonstrated that the metastable phase was thermodynamically stable, identified the 3D structure of metastable-phase crystals, and suggested a new nanoparticle growth mechanism called “multi-stage crystallization.”
|
|
This study holds significance as it reveals a new paradigm in metastable-phase-based material development when most research is focused on developing stable-phase materials.
|
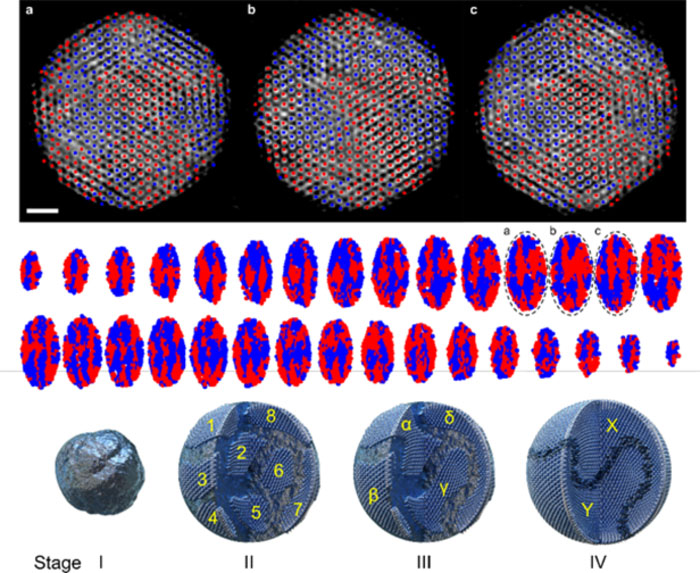 |
| 3D atomic structure of metastable palladium hydride nanoparticles as identified by atomic electron tomography and a schematic of the metastable-phase nanoparticle growth process. (Image: Korea Institute of Science and Technology)
|
|
Dr. Chun emphasized that “These study findings provide an important process to obtain source technology in the development of advanced alloy materials containing lightweight atoms. An additional study is expected to reveal a new paradigm in the development of metastable-phase-based eco-friendly energy materials that can store hydrogen and lithium. Similar to the Czochralski (CZ) method, which is used to produce single-crystal silicon, a key material in today’s semiconductor industry, it will be a source technology with great potential that will contribute to advanced material development.”
|



