| May 25, 2022 |
Crystallization of nickel out of liquid observed in real-time at the atomic level
(Nanowerk News) The potential use of nickel in new nanomaterials and as a low-cost catalyst in chemical reactions serving a range of industrial processes has been held back by a limited understanding of fundamental aspects of how the metal crystallizes into a solid. Researchers however have now been able to observe such crystallization of nickel’s two structural forms at the atomic scale using liquid-phase electron microscopy.
|
|
A paper describing their observations appeared in the journal Nano Research ("Atomic mechanisms of hexagonal close-packed Ni Nanocrystallization revealed by in situ liquid cell transmission electron microscopy").
|
 |
| Researchers however have now been able to observe such crystallization of nickel’s two structural forms at the atomic scale using liquid-phase electron microscopy. (© Nano Research)
|
|
Catalysts—any substance that speeds up a chemical reaction speed—are essential for the production of a myriad of industrial products, but one of the challenges that their use in a range of purposes, not least energy technologies, is that many are precious metals. Platinum for instance, which costs hundreds of dollars an ounce, is used as a catalyst to accelerate reactions sufficient to make a range of clean fuel sources viable.
|
|
Nickel on the other hand happens to be one of the most abundant metals in the earth’s crust, and as such costs just pennies an ounce. Nickel is also extremely stable in a variety of environments. As a result, nickel-based catalysts have recently received considerable research attention due to their diverse catalytic applications. But precious metals-containing catalysts give a greater boost to the speed of reactions than nickel.
|
|
A range of strategies have been developed to improve nickel’s catalytic capability, and to deploy nickel as a component of new nanomaterials, but for still greater progress to be made, researchers need to do a better job of understanding some of the most fundamental aspects of how nickel forms and its structure as it does so.
|
|
To perform such investigations, they explore nickel crystals at their very tiniest state, at the beginning of their formation (or ‘nucleation’) out of a liquid. These are called nanocrystals – any crystal particle with at least one of its sides measuring less than 100 nanometers (one thousand-millionth of a meter).
|
|
Nickel nanocrystals take two crystal-lattice forms: a cubic structure and a hexagonal one, called “hexagonal-close-packed” or hcp. Understanding the mechanism of how these two lattice structures emerge – the crystallization process – has remained largely unknown. To achieve the deep understanding of the crystallization process desired would require a direct observation in real time of the nucleation pathways of hcp nickel nanocrystals at the atomic level.
|
|
Other researchers had achieved success in real-time imaging of the crystallization pathways of silver and gold nanocrystals using liquid-phase electron microscopy, revealing the multi-step nucleation mechanisms of the crystal formation of these elements.
|
|
Electron microscopy involves the use of a beam of electrons to illuminate an item of interest rather than photons as with a normal microscope. This is because an electron’s wavelength is far smaller than that of the photons that make up visible light, allowing for investigation of extremely tiny objects.
|
|
Liquid-phase electron microscopy involves the same process, but permits the observation of specimens in liquid. As the target is precisely how solid crystals emerge out of a liquid, liquid phase electron microscopy has been a powerful tool for observing such nucleation and growth of nanocrystals.
|
|
“In principle, the Ni nanocrystals could crystallize in fcc or hcp phases. Usually, the formation of the new phase of nanocrystals is dependent on the adsorption energy of surfactant and the surface energy of exposed facets. Some researchers had earlier used this technique to investigate the formation of the cubic structural form of nickel nanocrystals in homogeneous Ni(II) growth solution containing Ni-ammine-acetate complexes,” said Junyu Zhang, a co-author of the paper and researcher with the Instrumental Analysis Center at Huaqiao University. “And now, in this work, both in situ liquid cell TEM study and theoretical calculations identified the non-classical features of hcp Ni crystallization in N,N-Dimethylformamide (DMF) solution at a high dose rate of the electron beam.
|
|
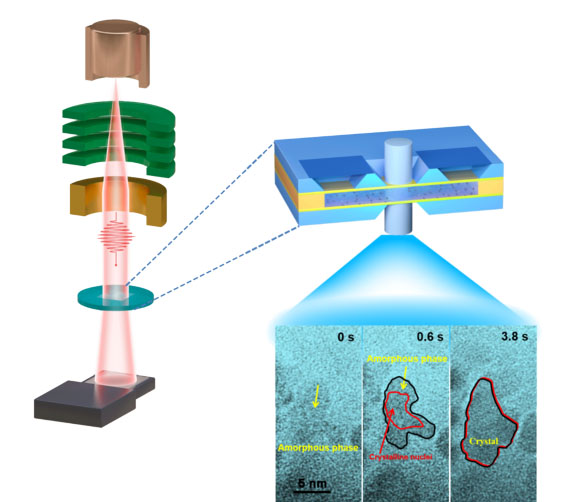
The researchers mixed a liquid solution that contained more nickel than could be dissolved (a ‘supersaturated solution’), thus any excess would naturally precipitate out as a solid (in other words via crystallization). They then used the liquid-phase electron microscope to observe in real time the nucleation.
|
|
Specially, they have reported the real-time and direct visualization of the dynamic processes of amorphous-phase-mediated crystallization of Ni hcp nanoparticles with (10) or hcp Ni (0001) facets in a homogeneous solution through spinodal decomposition, solidification, and atomic-scale crystallization under high dose rate of electron beam. They also imaged the facet development of Ni nanocrystal by layer-by-layer growth. Finally, the unstable Ni dissolved into the solution.
|
|
Armed with an understanding of the fundamental stages of the formation of nickel crystals at their tiniest size, the researchers believe that this could provide unique insight for the future design of hcp-nickel materials systems and catalysts.
|

