| Nov 18, 2022 |
Novel technique to synthesize platinum single-atom catalysts with ultrahigh mass activity
(Nanowerk News) Hydrogen is a promising clean energy carrier due to its highest gravimetric energy density and zero carbon dioxide emissions. The noble metal Pt is the most effective catalyst for electrochemical water splitting to produce hydrogen. However, the rarity and high cost of Pt severely limit its practical application.
|
|
Recently, a research group led by Prof. ZHU Qingshan from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences has developed a cation defect engineering technique to synthesize Pt-single-atom catalysts with ultrahigh mass activity for large-scale hydrogen production at low cost.
|
|
The study was published in Advanced Functional Materials ("Ultrahigh Mass Activity for the Hydrogen Evolution Reaction by Anchoring Platinum Single Atoms on Active {100} Facets of TiC via Cation Defect Engineering").
|
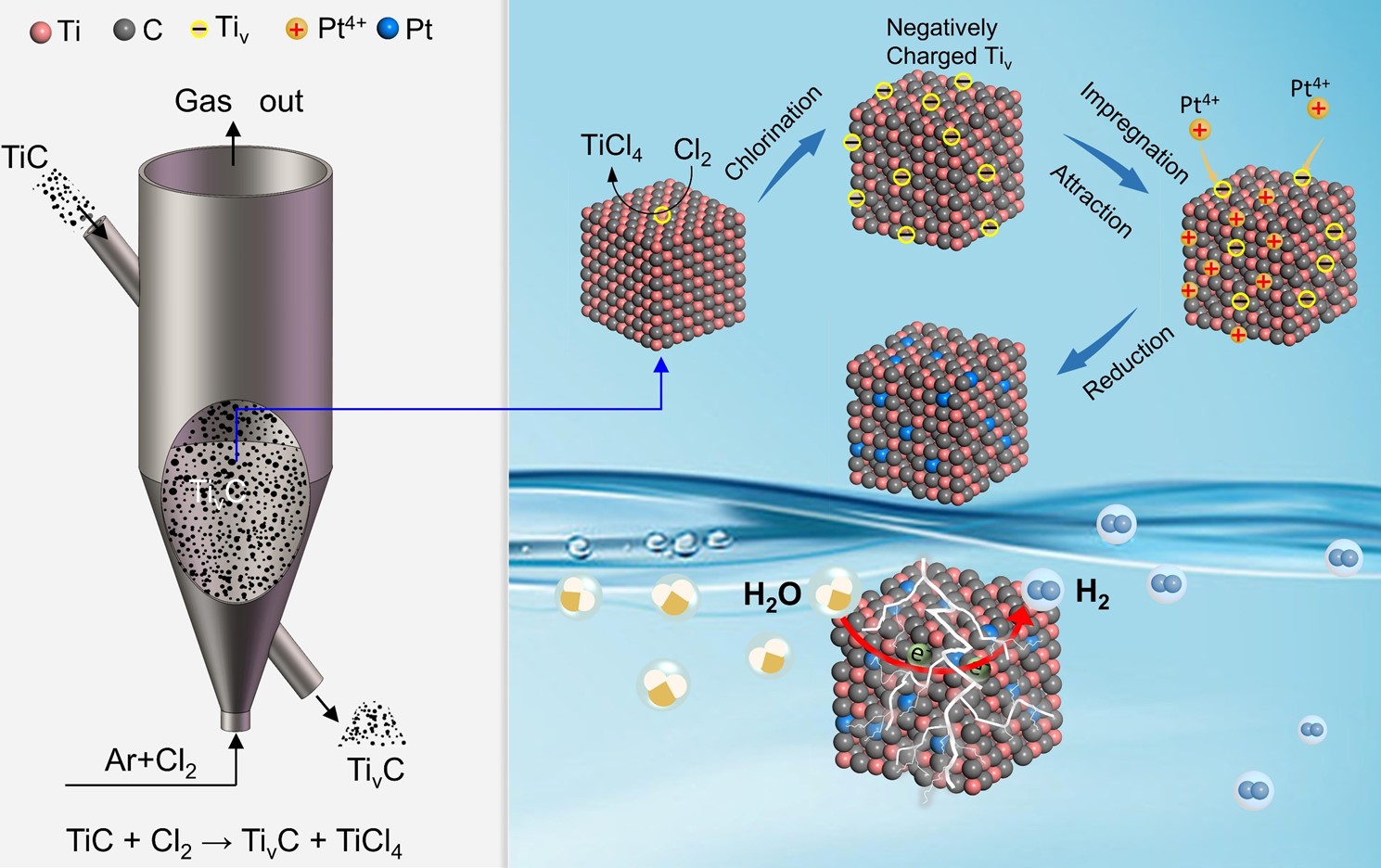 |
| Schematic diagram of the cation defect engineering fluidized technique. (Image by XIANG Maoqiao)
|
|
Over the past decade, space confinement, strong interaction, and functional group constraint strategies were developed to fabricate Pt single atom catalysts for maximumly utilizing Pt. However, the Pt mass activity has not been improved significantly.
|
|
One of the main reasons is that single atoms are thermodynamically unstable and tend to spontaneously aggregate into particles during the synthesis and operation, decreasing the mass activity.
|
|
In this study, the cation defect engineering technique can anchor platinum (Pt) single atoms on the active {100} facets of titanium carbide (TiC). The mass activity of the as-synthesized Pt-TivC single-atom-catalyst was approximately 190 times that of the commercial 40 wt% Pt-C catalyst, with low Pt loading amount and low cost.
|
|
"Ti atoms in the surface of active TiC {100} facets were selectively chloridized to form Ti vacancies with negatively charged, subsequently, Pt atoms were anchored in the Ti vacancies by forming covalent Pt-C bonds, showing excellent long-term durability and ultrahigh mass activity," said Dr. XIANG Maoqiao, co-corresponding author of the study.
|

