| Feb 05, 2014 |
Adaptable crystals allow quick, efficient separation of carbon monoxide from gas mixtures
|
|
(Nanowerk News) Researchers at Kyoto University have developed a new material capable of easily separating carbon monoxide (CO) from gas mixtures. Their report, published in the online edition of Science ("Self-Accelerating CO Sorption in a Soft Nanoporous Crystal"), could have large implications for bulk manufacturing and gas recovery in the chemical industry.
|
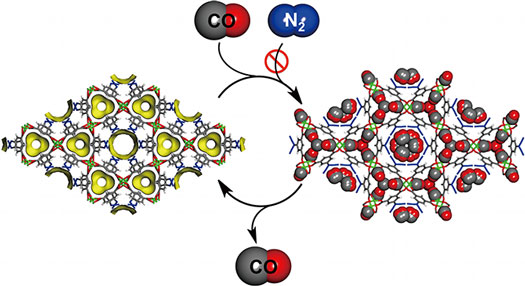 |
| A new nanoporous material (left) has been successfully shown to selectively adsorb carbon monoxide (CO) from a gaseous mixture, rejecting other gases such as nitrogen (N2). The captured CO can then later be released, returning the lattice to its empty state. (Graphic courtesy of the Kyoto University iCeMS Kitagawa Lab)
|
|
CO is an important industrial gas that is used as a building block for many chemical products. It is also a byproduct of industries such as steel manufacturing. Unfortunately, this source of CO is generally mixed with nitrogen and cannot be reused, eventually getting burned off in the form of carbon dioxide, a greenhouse gas. Current methods to salvage and separate CO from gas mixtures use metals that require high temperatures and hence large energy expenditure, resulting in great cost.
|
|
The team of investigators, from Kyoto University's Institute for Integrated Cell-Material Sciences (iCeMS) and School of Engineering, utilized their expertise in porous coordination polymer (PCP, also known as metal organic framework or MOF) crystals, cage-like structures with nano-sized pores and channels, to selectively purify CO with low energy usage.
|
|
"We designed copper-based PCPs to accomodate CO with two types of flexible channels," explained Hiroshi Sato, lead investigator of the study, "one open and the other narrowly constricted. When we added this PCP to a CO and nitrogen gas mixture, CO was taken up slowly at first, but as more and more of the gas entered into the crystals an overall structural change was triggered that widened the narrow channel and allowed for accelerated CO uptake."
|
|
The scientists confirmed that CO was selectively adsorbed by the PCP, and that the gas could later be released in a simple process. Repeating this resulted in a gas with 94% CO purity when the original mixture contained equal amounts of CO and Nitrogen.
|
|
"What is important for effective separation of CO is a very weak affinity between copper and CO gas, which allows for a reversible open and closed state of the channel," said Ryotaro Matsuda, a corresponding author in the report. "Our method can be applied to the separation of different gases using other PCPs."
|
|
According to Susumu Kitagawa, iCeMS' director and principal investigator of the study, "We hope this area of research can be applied to the efficient purification of other gases at greatly reduced cost. Moreover, we feel our findings could open up new, unexplored avenues utilizing these types of compounds together with various biomolecules."
|

