| Posted: Feb 02, 2011 | |
Nanostructured scaffolds offer a promising route to repairing spinal cord injuries |
|
| (Nanowerk Spotlight) Spinal cord injury in humans remains a devastating and incurable disorder. It is still a traumatic pathology that may impair patients' movements by interrupting their motor-sensory pathways. It is estimated that there are approximately 2.5 million people worldwide with a spinal cord injury and over 130,000 people each year survive such a traumatic injury, often bound to spend the rest of their lives in a wheelchair. | |
| Rapid progress in tissue engineering, especially electrospinning techniques that lead to micro- and nanofibrous flexible tubular scaffolds for nerve cell regeneration, may lead to promising therapies for spinal cord injuries. | |
| Researchers in Italy, led by Angelo Vescovi and Fabrizio Gelain, at the CNTE at Niguarda Ca'Granda Hospital, University of Milan-Bicocca and IRCCS Casa Sollievo Della Sofferenza, in collaboration with the Institute for Soldier Nanotechnologies at MIT, have now demonstrated the repair of a chronically injured spinal cord by attempting to replace the fluid-filled cyst found in these lesions with a neuroprosthetics conducive to tissue reconstruction and axonal regeneration. | |
 |
|
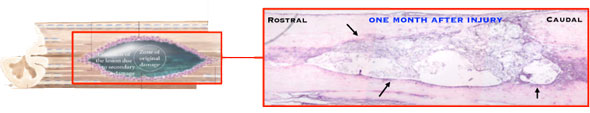
| Spinal cord injury. (Image: Fabrizio Gelain; American Chemical Society) | |
| "We managed, for the first time, to obtain a consistent regeneration of the nervous tissue in chronicized injuries at the spinal cord by using a nanostructured composite scaffold with no cells in it," Gelain tells Nanowerk. "Where usually in the damaged spinal cord there is scar tissue or a fluid filled cyst, nervous tissue regenerated and followed the direction given by our guidance channels. Hystological results were also supported by significant functional recovery of the treated animals." | |
| Reporting their findings in a recent issue of ACS Nano ("Transplantation of Nanostructured Composite Scaffolds Results in the Regeneration of Chronically Injured Spinal Cords"), the team provided the proof-of-principle that nanotechnology has the tremendous potential of offering nanostructured scaffolds that now must be considered as a new strategy among the already known set of promising approaches for spinal cord injury (cell therapy, rehabilitation, etc). | |
| "By themselves, nanostructured scaffolds probably will not solve the problem of regenerating chronic and acute spinal cord injury" says Gelain. "However, they will become a necessary component of an effective multi-disciplinary therapy in the near future. Moreover, we demonstrated that two different techniques like self-assembling and electrospinning can synergically work together to achieve the specific goal of central nervous system regeneration." | |
| Spinal cord injury in humans involves the permanent destruction of the nervous tissue. Within the affected regions, the mechanical substrates that provide physical support for axonal regeneration and three-dimensional positional information as well as the cytoarchitectural organization required for effective nerve regrowth have gone permanently lost. These holes in the spinal cord tissue represent an insurmountable barrier for axonal regeneration. | |
| Gelain points out that the most pressing issue in chronic spinal cord injury is to warrant a suitable level of anatomical, histological, and cellular reconstruction at the lesion site. "Thus, the scar tissue and hollow cysts should be replaced with new neural tissue, permissive for both axonal regrowth and lesion bridging." | |
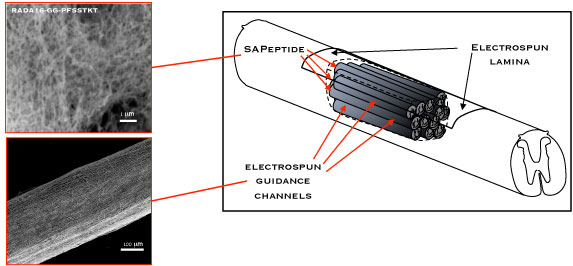
| To that end, the Italian team engineered neural prosthetics made of composite nanostructured synthetic scaffolds, constituted of functionalized self-assembling peptides injected into electrospun nanostructured polymeric guidance channels, loaded with pro-regenerative drugs. They then implanted these guidance channels into cysts (cavities) of the damaged spinal cord of rats. | |
 |
|
| Nanostructured composite scaffold as guidance channels. (Image: Fabrizio Gelain; American Chemical Society) | |
| Over the past few years, Gelain and his team have done a lot of pioneering work in this area. They developed a new set of functionalized self-assembling peptides specifically to obtain nervous regeneration ("Designer Self-Assembling Peptide Nanofiber Scaffolds for Adult Mouse Neural Stem Cell 3-Dimensional Cultures"), optimized their design, and tested the release of cytokines in vitro from these self-assembling peptides ("Slow and sustained release of active cytokines from self-assembling peptide scaffolds"). | |
| "We optimized the design of electrospun guidance channels testing their potential in sciatic nerve regeneration ("Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections") prior to undertaking our expensive and time-consuming experimentation – a complex work necessary for the design of our scaffolds" says Gelain. | |
| What the team found during their experiments is that the implanted tubular channels indeed were able to 'guide' regenerating nervous fibers across the implants, thereby crossing the cysts. | |
| "When implanted in the chronically injured rat spinal cord, the neuro-prosthetics underwent gradual bio-degradation causing negigible inflammatory response" says Gelain. "The nanostructured scaffolds led to the cyst being replaced by newly formed tissue, composed of both neural, vascular and stromal (support) cell types, that provided the appropriate environment for axonal regeneration and myelination, electrophysiological improvement and significant neurological recovery." | |
| This is the first evidence of nanostructured composite synthetic scaffold producing broad histological, vascular and cyto-architectural reconstruction, yielding to spatially guided nervous regeneration and neurological recovery in chronic injuries of the spinal cords. | |
| This innovative study opens a new branch of investigation in the field of biomaterials and development of novel therapeutics for chronic spinal cord injury in combination with other approaches like cell therapy and drug release. | |
| The team is already working on improving their scaffold by adding cells – they are already testing GMP-grade human neural stem cells – in order to obtain even more significant results. Then, the next step will be to move on other mammalian animal models and, finally, to begin clinical studies. | |
| "Since electrospinning and self-assembling are two extremely versatile technologies, we believe that our work will inspire other researchers to target other tissues by tuning and adapting our approach," says Gelain. "For example we think of testing the regenerative potential of these composite scaffold in the regeneration of skin and cartilage." | |
| He notes, though, that one of the remaining main challenges is on of scientific cooperation. | |
| "An effective therapy must be multi-disciplinary and, to be performed at the maximum of its potential, need the contribution of research groups dedicated to each facet of the problem. We are a multi-disciplinary group, but there are limitations that can only be solved with whole teams dedicated to each part of the project." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
