| Posted: Oct 16, 2017 | |
Nanotechnology innovation safety aspects - the case of graphene |
|
| (Nanowerk Spotlight) Currently, most graphene-based innovations are not yet at the level of large-scale commercial production. But public and private investments into graphene and its applications in products are large and whichever production methods eventually turn out to be successful, exposure to humans or the environment somewhere along the value chain or life-cycle of the material or product should be anticipated timely. | |
| The number of scientific papers on graphene now exceeds 12000 per year and commercial interest is equally large, reflected by a total of almost 21000 patents worldwide since 2000. Graphene is expected to be the focus of even greater interest for industrial applications when mass-produced graphene will have the same outstanding performance as the best samples produced in research laboratories. So far, however, researchers have been unable to produce large quantities of high-quality graphene as no scalable production method exists. This has been the subject of ongoing international research. | |
| Authors of a review paper in ACS Nano ("Considerations for Safe Innovation: The Case of Graphene") believe that it is exactly at this early innovation stage that one should be made aware of potential risks. By offering suggestions on how potential nanotechnology specific safety issues can be addressed, by who and at what stage of the innovation process, they hope to have provided guidance on what investments are needed to market safe graphene-based products. | |
| Analogous to properly considering the risk factors of nanomaterials in general, if innovators, scientists and regulators alike take the necessary actions, this should lead to a more efficient innovation process and increase the chances of success in yielding safe applications of graphene. | |
| The innovation process of graphene may serve as an example that would benefit from considering safety aspects at various stages of development, according to a similar strategy as suggested for nanomaterials in general. | |
| If safe innovation aspects of graphene are not taken into account timely, uncertainty about the safety of production, use, and waste handling of graphene-based products remains, which may impact their commercial success, even for products that are already on the market. | |
| The aim of this review paper is to outline what safety aspects can be considered in the “potential”, “indicator”, and “demonstrator” phases of the innovation process of graphene. The authors from the Netherlands National Institute for Public Health and the Environment (RIVM) provide an overview of available information for assessing hazard, exposure, and risks and, where relevant, indicate further steps to be taken by various stakeholders to promote the safe production and use of graphene. | |
| After a detailed discussion of nomenclature and classification; characterization; production; and applications of graphene, the authors delve into the issues of human exposure to graphene and its environmental fate. | |
 |
|
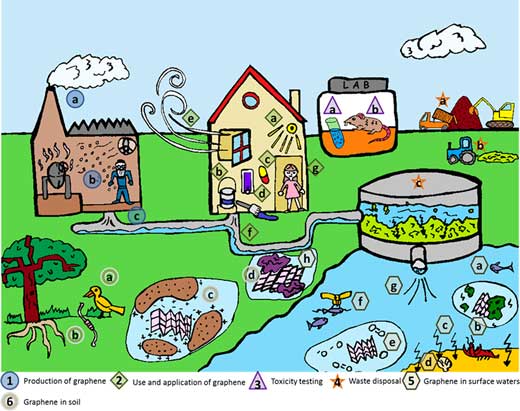
| Life cycle of graphene showing factors relevant to consider in a safe innovation approach. (1) Production of graphene: (a) emission to air during production processes; (b) occupational exposure assessment; (c) emission to wastewater during production processes. (2) Use and application of graphene: (a) sensor technology; (b) coatings and paints; (c) drug delivery; (d) information technology; (e) emission to air during consumer use; (f) emission to wastewater during use; (g) consumer exposure assessment; (3) toxicity testing; (a) in vitro toxicity; (b) in vivo toxicity. (4) Waste disposal: (a) agricultural application of wastewater sludge; (b) wastewater treatment. (5) Graphene in surface waters: (a) environmental exposure estimation of organisms in surface water; (b) attachment to naturally suspended particles; (c) settling from the water column to the sediments at the bottom; (d) environmental exposure estimation of organisms in sediment; (e) oxidation of graphene; (f) bioaccumulation via the food chain; (g) emission from wastewater treatment; (h) sorption of other pollutants to graphene in water. (6) Graphene in soil: (a) bioaccumulation via the food chain; (b) terrestrial exposure; (c) retention; (d) sorption of other pollutants to graphene in soil. (Reprinted with permission by American Chemical Society) (click on image to enlarge) | |
| Exposure to graphene could occur during the entire life cycle from production to waste (see figure above). Potential exposure of workers, i.e., occupational exposure, may be relevant during all stages of product development, while direct exposure of consumers (including sensitive subpopulations) will only occur when handling finished products. | |
Occupational exposure to graphene |
|
| In a NIOSH survey report (pdf) on engineering controls for graphene platelets during manufacturing and handling processes, the release of particles in the manufacturing location during several phases of graphene production was monitored, and the effectiveness of several control measures such as canopy hoods and exhaust ventilation were evaluated. However, the type and effectiveness of potential exposure reducing measures will depend on the scale at which graphene is produced. | |
| At present, as graphene applications are mostly still under development, the main human exposure of concern forms the respiratory exposure of workers during production and handling in graphene production facilities. Although production volumes will likely increase as one moves through the different product development stages, this does not necessarily imply that exposure levels will also increase as manufacturers may have more information available on the potential exposure pathways of their product and may also have more resources available to implement exposure reducing measures. | |
| The level of exposure to graphene will largely depend on the production method and the exposure reducing measures during production. During use of (future) applications of graphene, the material will likely be enclosed in or fixed to a product, although migration of graphene from finished products may still occur. | |
| Next to assessing the exposure to graphene itself, it is of importance to consider exposure to impurities that may be released during the production of graphene or impurities that remain associated with the final graphene product, e.g., absorbed to the surface. | |
Environmental fate of graphene |
|
| Similar to other engineered nanomaterials, it is expected that releases of graphene to the environment will take place during all stages of the life cycle of graphene containing products. However, compared to other nanomaterials, very limited information is available to perform proper fate assessment of graphene in the various environmental compartments (soil, water, air). | |
| The only possible way to effectively reduce environmental exposure to graphene is to reduce its emission to the environment. Following introduction in solid matrices like soils and sediments, graphene will persist and solid-phase concentrations are expected to increase linearly over time at equal emissions. Once graphene is introduced into an aquatic environment, it will transform to graphene oxide (GO) and remain present as a persistent colloidal suspension that over time will settle and induce sediment contamination. | |
| In wastewater treatment plants, colloids will be formed similarly, and these will settle out in the sludge but still remain intact. Application of sludge from wastewater treatment plants to agricultural lands should be avoided, since persistent nanomaterials such as GO in the sludge may wash out and reach surface waters. Alternatively, wastewater from graphene production should not be introduced in municipal wastewater treatment plants at all but treated directly at the site of production. | |
Hazard evaluation of graphene |
|
| While there is little concern for graphene sheets that are securely embedded within the matrix of a product, graphene may be released either during production or from the matrix of the product during its life cycle and thus it is important to evaluate their potential hazardous properties. Contradictory results have been reported in the literature on the toxicity of graphene-related materials. This is mainly because of the significant variability of the materials used in toxicity testing and differences in experimental setups. | |
| Based on the current knowledge on hazards of nanomaterials, a number of properties can be identified that may be relevant for hazard assessment of graphene. The authors note that, as with regular chemicals, potential hazards of graphene may be caused by graphene itself but also by materials resulting from its potential biotransformation, such as enzymatic activation. | |
| However, very little knowledge is available on the biotransformation of graphene and the potential hazards resulting from this, even for nanomaterials in general, and this is therefore not considered further in the review. | |
Safety-related regulations of graphene |
|
| The OECD Council recommended Members to apply the existing international and national chemical regulatory frameworks or other management systems to manage the risks of manufactured nanomaterials. In line with this recommendation, most countries indeed try to manage the risks of manufactured nanomaterials in their chemicals legislation, e.g., the REACH Regulation in Europe and TSCA in the U.S. | |
| In view of its small dimensions, graphene is specifically mentioned in the EU Recommendation for a definition of a nanomaterial. As a result, graphene is specifically mentioned in those legislations that fully incorporated this definition (e.g., the biocidal products regulation). This definition, including the specific mentioning of graphene, is also incorporated in the recently published new Medical Device Regulation. In contrast, while the definition of a nanomaterial is included in the Novel Food Regulation, graphene is not specifically mentioned here. | |
| Concluding their review, the authors suggest a number of approaches to be taken by different stakeholders (innovators, scientific experts, risk assessors, and regulators) at different stages of innovation to optimize a safe innovation process of graphene and graphene based products. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
