| Posted: Feb 09, 2018 | |
Controlling the kinetics of ion-capturing/ion-releasing regimes in liquid crystals by means of nanoparticles |
|
| (Nanowerk Spotlight) Liquid crystal devices such as laptops, TV screens, mobile phones, smart watches and tunable lenses are ubiquitous in daily life. Most of these liquid crystal displays have one thing in common: they are driven by electric fields (recall how critical is to check the battery of your laptop prior to doing an important work). | |
| An ideal operation of the afore-mentioned liquid crystal devices assumes that liquid crystals do not conduct electricity and behave as anisotropic dielectric liquid. This means the device is based on the so-called field effect when the voltage applied across the liquid crystal layer re-orients elongated liquid crystal molecules along the electric field. | |
| The reorientation of liquid crystals under the action of the applied electric field results in changing the optical properties of liquid crystal devices (for example, we can switch between bright and dark states of the image). | |
| This electric-field induced driving of liquid crystal devices can be severely altered by ions typically present in liquid crystals. Even small traces of ions in liquid crystals can lead to the so-called screening effect, causing such negative things as image sticking, image flickering, and overall slow response of the device. | |
| All these negative effects compromise the performance of liquid crystal devices. That is why the reduction of the concentration of ions in liquid crystals is among the grand challenges facing the modern liquid crystal industry, spanning display and emerging non-display applications. | |
| One promising way to solve this challenge can be found by merging nanotechnology and liquid crystals. In short, nano-objects immersed in liquid crystals can capture ions thus reducing the concentration of mobile ions and improving the performance of liquid crystal devices. | |
| This idea has been tested by many research groups around the globe (for more detail please refer to a recent review paper in Crystals: "Nano-Objects and Ions in Liquid Crystals: Ion Trapping Effect and Related Phenomena" ). | |
| Indeed, existing experimental and theoretical studies indicate a high promise of nanomaterials to provide a permanent purification of liquid crystals from ions. In other words, under certain conditions nanomaterials can act as ion capturing agent in liquid crystals. | |
| In previous Nanowerk Spotlights we have already discussed some tricky behavior of nanomaterials and ions in liquid crystals: | |
|
|
|
| So far, the majority of existing reports on the behavior of ions and nanomaterials in liquid crystals were focused on the steady-state ion capturing properties. In other words, these studies were performed assuming the equilibrium state is reached. | |
| A very practical question is the kinetics of ion-capturing/ion-releasing processes in liquid crystals doped with nanomaterials. | |
| "Imagine, we want to purify liquid crystals from ions using nanomaterials," says Yuriy Garbovskiy, PhD, a researcher at the UCCS BioFrontiers Center & Department of Physics, University of Colorado. "We mix our nanomaterials with liquid crystals. How much time is required to achieve the expected ion capturing effect? Should we wait for an hour or for a month? Moreover, can we control this time by any means?" | |
| Garbovskiy has provided an answer to this set of important practical questions in a paper published in Nanomaterials ("Kinetics of Ion-Capturing/Ion-Releasing Processes in Liquid Crystal Devices Utilizing Contaminated Nanoparticles and Alignment Films"). | |
| This paper provides an analysis of the kinetics of ion-capturing/ion-releasing regimes in liquid crystals doped with nanoparticles. | |
| A very important aspect of this study is an explicit consideration of the effects of the ionic contamination of nanoparticles on the kinetics of ion-capturing/ion-releasing effects in liquid crystal nanocolloids. | |
| "In short, 100 % pure nanoparticles dispersed in liquid crystals result in the ion-capturing regime," explains Garbovskiy. "However, if nanoparticles are contaminated with ions prior to dispersing them in liquid crystals, three different regimes are possible, namely, the purification regime (nanodopants capture ions in liquid crystals); ion releasing regime (liquid crystals are enriched with ions desorbed from the surface of nanoparticles); and no change regime (nothing happens)." | |
| Typical examples of these regimes is shown in the figure below. | |
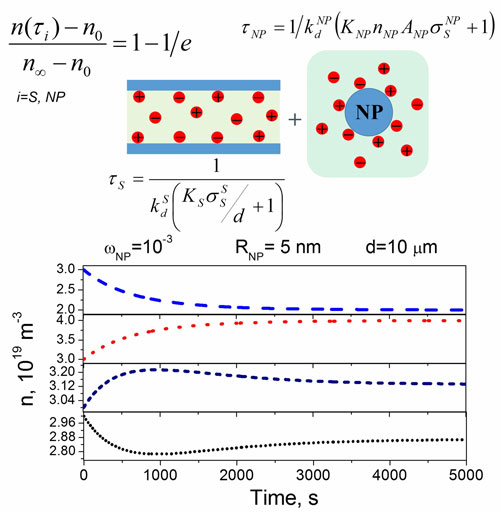 |
|
| Ion-capturing/ion-releasing regimes in liquid crystals doped with nanoparticles as a function of time. Ion capturing regimes are represented by dashed (blue) and short-dotted (black) curves while ion releasing regimes are shown as dotted (red) and short-dashed (navy) curves (Source: MDPI, Nanomaterials, DOI:10.3390/nano8020059, Creative Commons Attribution License) | |
| Another important aspect of this paper is the consideration of the combined effect of both nanoparticles and substrates on the kinetics of ion-capturing/ion-releasing regimes in liquid crystals – recall, in all devices liquid crystals are sandwiched between two substrates so the consideration of interactions of ions with substrates is equally important. | |
| As a result, the kinetics of ion-capturing/ion-releasing process in liquid crystals doped with contaminated nanoparticles and sandwiched between two substrates is characterized by several time constants. These time constants originate from the presence of nanoparticles and substrates. | |
| "An interesting thing is that these time constants can be controlled by changing the concentration of nanoparticles, their size, the type of ion capturing materials, and the thickness of the liquid crystal cell," Garbovskiy points out. "For example, we can shorten time needed to achieve a steady-state by increasing the concentration of nanoparticles and decreasing their size. As a result, it enables the control over the kinetics of ion-capturing/ion-releasing process in liquid crystal nanocolloids by changing the afore-mentioned parameters." | |
| The results presented by Garbovskiy in this work are very important for scientists and R&D engineers trying to transition the concept of nanoparticle-enabled control of ions in liquid crystals from academic to industrial domain. | |
| In addition, this paper highlights the importance of considering the purity of both nanoparticles and substrates used in liquid crystal devices. | |
| Finally, the presented results due to their quite general nature can guide the selection of the most efficient and very 'fast' ion-capturing nano-objects. | |
| "From a more general perspective, the presented results can enable emerging applications of liquid crystals doped with nanoparticles such as microwave devices and flexible electronics," Garbovskiy concludes. "As a result, future research directions are very broad and can include extensive experimental studies of time-dependent ion-capturing/ion-releasing properties of various types of nanomaterials, liquid crystals and alignment layers." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
