| Dec 02, 2019 | |
Transforming the greenhouse gas carbon dioxide into graphene |
|
| (Nanowerk Spotlight) In previous Nanowerk Spotlights we have reported on research efforts by Stuart Licht, a professor of chemistry at George Washington University, that use molten carbonate chemistry to transform atmospheric carbon dioxide (CO2) into valuable nanomaterials (read more: "'Diamonds from the sky' chemistry converts greenhouse gas into valuable nanocarbon materials"). | |
| In this process, which Licht termed C2CNT (carbon dioxide to carbon nanotube), CO2 is split into its constituent components carbon and oxygen by molten carbonate electrolysis. | |
| The molten electrolysis process is similar to the industrial process in which aluminum is produced from its oxide (aluminum oxide, bauxite), although here another oxide, carbon dioxide, is the raw material and carbon nanomaterials are produced. | |
| More recently, Licht's group has demonstrated that when transition metal nucleating agents are specifically excluded from the C2CNT process, carbon nanotube growth is inhibited and other uniform carbon nanomaterials can be synthesized. Specifically, valuable carbon nanomaterials including carbon nano-onions and graphene can be produced at high yield by solar-powered molten carbonate electrolysis (Accounts of Chemical Research, "Recent Advances in Solar Thermal Electrochemical Process (STEP) for Carbon Neutral Products and High Value Nanocarbons"). | |
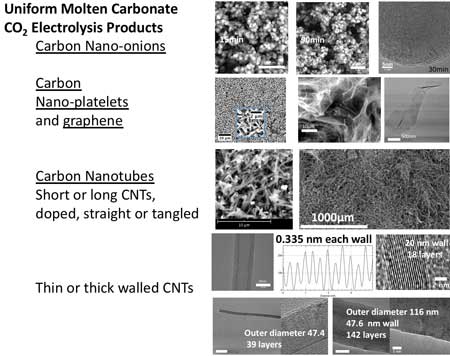 |
|
| A diverse range of uniform carbon nanomaterial products can be synthesized by CO2 electrolysis in molten carbonates including carbon nano-onions (shown after 15-90 minutes of electrolysis), carbon nanoplatelets, graphene, and a variety of carbon nanotubes. (Reprinted with permission by American Chemical Society) (click on image to enlarge) | |
| As described in Journal of CO2 Utilization ("Transformation of the greenhouse gas carbon dioxide to graphene"), Licht's team produced graphene by molten carbonate electrolytic splitting of CO2 to a nano-thin carbon product (carbon nanoplatelets) comprised of 25 to 125 graphene layers, and subsequent electrochemical exfoliation of the nanoplatelets to graphene in a carbonate soluble aqueous solution. | |
| "The sole products of the carbon dioxide electrolysis are straightforward: high yield carbon nanoplatelets and oxygen," Licht explains to Nanowerk. "The carbon nanoplatelets provide a thinner starting point than a conventional graphite reactant to facilitate electrochemical exfoliation." | |
| The graphene produced in this way has a thickness of 1–5 carbon layers, but a relatively small lateral dimension of 2–8 µm. This lateral size is for example of use in graphene as a lubricant, in battery anodes, and in graphene admixture applications. | |
| As shown in the figure below, electrochemical exfoliation of graphene is a process in which intercalated ions between graphite layers are oxidized, forming gases which break apart the interlayer bonds, and release graphene sheets. | |
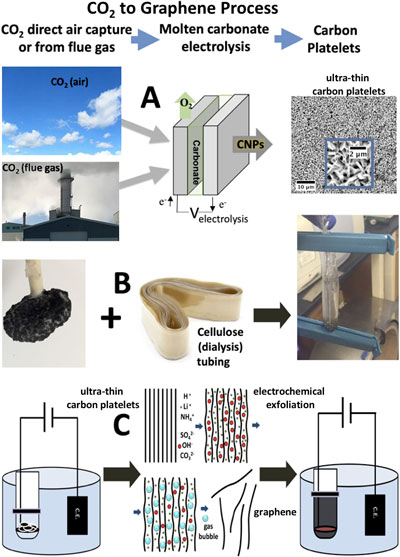 |
|
| Electrosynthesis of graphene from CO2. A: CO2 from the air or from flue gas is electrolytically split to ultra-thin graphene platelets product by molten carbonate electrolysis. B: The carbonate synthesis cathode containing product is cooled and placed in a cellulose tube containing aqueous (NH4)2SO4. C: The cellulose tube is placed in an (NH4)2SO4 bath with a counter electrode; DC voltage is applied that generates gas bursts between the graphene layers exfoliating the thin platelets producing graphene. (Reprinted with permission by Elsevier) (click on image to enlarge) | |
| "The molten electrolysis of this CO2 process is unusual in that it sustains removal of the greenhouse gas not only from concentrated streams, such as industrial flue gases, but also – without the need for preconcentration or purification – directly from the air," Licht points out. "In addition, the strong graphene bonds of the carbon nanomaterials can permanently, i.e. over a geologic time frame, store the removed CO2, as opposed to fuel products which again release the CO2 when the fuels are consumed." | |
| As described at C2CNT.com and CarbonXPrize.org, Licht's team is scaling the electrolysis of CO2technology as a finalist in the international Carbon XPrize competition at their 2-5 tonne a day CO2 transformation plant (see: "Transformative Carbon XPrize technological advance in CO2 utilization"). | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
