| Nov 22, 2021 | |
Appearance and origin of oxidation in 2D nanomaterials akin to tree rings |
|
| (Nanowerk Spotlight) Transition metal dichalcogenides (TMDs) represent a large family of layered semiconductor materials of the type MX2, with M a transition metal atom (Mo, W, etc.) and X a chalcogen atom (S, Se, or Te). One layer of M atoms is sandwiched between two layers of X atoms. Many TMDs exhibit tunable band gaps that can undergo a transition from an indirect band gap in bulk crystals to a direct band gap in two-dimensional (2D, i.e. monolayer) nanosheets. (e.g., MoS2, WSe2, WS2, MoTe2). | |
| Due to their extraordinary optical and electrical properties, these 2D TMDs have emerged as a promising class of atomically thin semiconductors for a new generation of electronic and optoelectronic devices. For instance, TMDs possess optical properties that could be used to make computers run a million times faster and store information a million times more energy-efficiently. | |
| Recent studies also have shown the impressive photo-current conversions of these atomically thin semiconductors, such as MoS2 nanosheets, making them great candidates for next-generation visible light photodetectors. | |
| However, many 2D materials with atomic-scale thicknesses suffer from oxidation and degradation effects under ambient conditions, which is one of the biggest obstacles in their practical applications. Given all the excitement around 2D TMDs, one particular issue that has garnered interest in the TMDs research community is the long-term stability of these materials in different conditions. | |
| They must, for instance, be entirely shielded from light, with even short exposure causing oxidation severe enough to damage electrical contacts and completely destroy optical characteristics. | |
 |
|
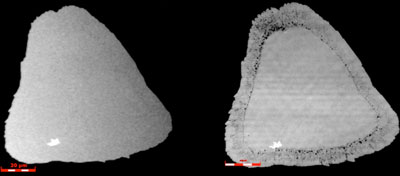
| Monolayer tungsten disulphide (WS2) is damaged by light. (Image: ARC Centre of Excellence in Future Low-Energy Electronics Technologies) | |
| In particular, the edges of TMD crystal flakes are susceptible to degradation if they are not protected by other layers. | |
| "When assembling van der Waals heterostructures, it is very useful to take the layer order and location of protected and unprotected edges in the TMDs flake into consideration, when for instance placing contacts or planning experiments involving multiple thicknesses," Peter Bøggild, a professor in the Department of Physics at Technical University of Denmark (DTU), tells Nanowerk. | |
| In their new work published in Nanoscale ("Long-term stability and tree-ring oxidation of WSe2 using phase-contrast AFM"), Bøggild and his colleagues show that phase-contrast atomic force microscopy (AFM) imaging allows to precisely distinguish covered from exposed edges in multilayer flakes. | |
| What is remarkable in this work is the fact that the research team's investigation covered a period of 75 months – so far the longest degradation study performed on any layered material. This enabled them to collect a unique data set of natural oxidation data in ambient conditions spanning this long time period of more than six years. | |
| "It was a bit of a coincidence that we started studying this flake over so many years," says Lene Gammelgaard, the paper's first author. "First, we wanted to verify the correlation between the optical contrast and the thickness of the flake. A staircase-like flake with multiple regions with different number of layers was ideal for this. However, when we observed the quite striking difference in the phase contrast for some of the steps we got excited and decided to look further into it; so we made sure to put the flake into dark storage for further study." | |
| That further study didn't take place for almost five years. Only then, when the researchers captured new AFM images of the previously exfoliated flake, they observed complex ring-like structures that not just in appearance but also in origin are akin to tree rings occurring in nature. | |
| Even more surprising, the team hypothesized that this tree-ring like structure could be correlated to the large variations in the Danish weather and that the distinct ring features are caused by variations and possibly combinations of environmental factors similar to real tree rings. | |
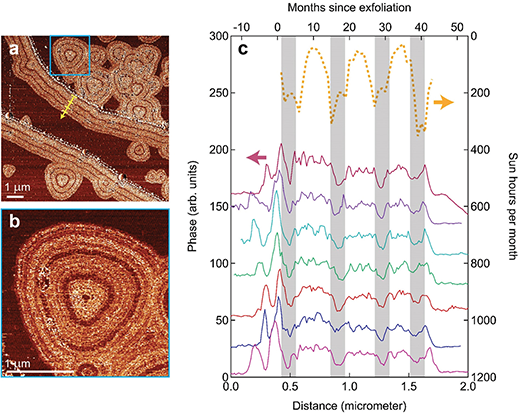 |
|
| (a) Phase contrast AFM image showing 4 clear lines emerging from the edges, and 1–4 lines from the point defects (triangular shapes). The yellow arrow shows the direction of the line-scans, with the 4 cross-bars indicating the approximate position of the bands with reduced phase-contrast. The region in the blue square is magnified in panel (b). The dark dot in the center of the tree-ring structure corresponds to the time where the oxidation process commenced. For radial (triangular) oxidation patterns process may start at a later time than for the edges, leading to smaller, partially complete oxidation patterns, yet with similar tree-ring structure. (c) 7 traces of phase-contrast AFM recorded in different locations of panel a, with dark bands indicating the four consistent minima, observed in panel a and b. On the right axis the sun hours per month are plotted against month (top axis). The maxima in sun hours (appearing as dips) corresponds roughly to the bands with minima in phase-contrast, until the sample was relocated to a dark storage container (M40). (Reprinted with permission by The Royal Society of Chemistry) (click on image to enlarge) | |
| "This study is definitely on the quirky side, but that being said, the long-term behavior of 2D materials in different conditions is really important," Bøggild notes. "What people often do is to accelerate the oxidation by for instance high temperature. These studies certainly have merit, but at least we are not convinced how well that compares to a real-time, ambient evolution, in our case a 75-month period." | |
| The team saw some strange features in the tree rings that were new to them, and which they have not encountered in literature, such as for instance Ostvald Ripening, a well-known process where smaller particles lose material to larger particles that then end up with uniform sizes. | |
| "We see a size distribution with two distinctly different sizes, ordered in a strange pattern, and we have no idea which microscopic mechanism(s) can result in such structures," Bøggild points out. "So, oxidation may be more complicated than you should think." | |
| "Our wonderful Center for Nanostructured Graphene is closing next year, and there is some poetry in that a part of its history the past many years has been engraved into a two-dimensional crystal, and in such a beautiful way," he concludes. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
