Transition Metal Dichalcogenides (TMDs) – A Promising Class of 2D Materials for Advanced TechnologiesContents
|
|
| Transition metal dichalcogenides (TMDs) are a class of two-dimensional (2D) materials with unique electronic and optical properties. Composed of transition metal atoms (such as tungsten, molybdenum, and selenium) and chalcogenide atoms (like sulfur or selenium), they have attracted significant attention in recent years for their potential applications in electronics, optoelectronics, energy conversion and storage, and more. | |
| The single-layer nature of TMDs, tunable bandgap, and large intrinsic carrier mobility make them ideal for use in various advanced technologies. | |
Structure and Properties of Transition Metal Dichalcogenides |
|
| TMDs are part of a large family of layered semiconductor materials, represented by the formula MX2, where M is a transition metal atom (Mo, W, etc.) and X is a chalcogen atom (S, Se, or Te). These materials consist of one layer of M atoms sandwiched between two layers of X atoms. | |
| Many TMDs, like MoS2, WSe2, WS2, and MoTe2, exhibit tunable bandgaps that transition from indirect to direct when transitioning from bulk crystals to 2D nanosheets. This allows for modification of the bandgaps by varying the number of layers. As the atomic layers decrease, the bandgap widens due to the quantum confinement effect, leading to a crossover from an indirect gap in bulk materials to a direct gap at the single-layer limit. | |
Van der Waals Heterostructures and Applications |
|
| The van der Waals interactions between neighboring layers of TMDs enable more flexible integration of different materials without lattice matching limitations, creating vast possibilities for controlling various properties at the atomic scale. These structures are called van der Waals heterostructures. | |
| Due to their extraordinary optical and electrical properties, 2D TMDs have emerged as a promising class of atomically thin semiconductors for next-generation electronic and optoelectronic devices. For example, their optical properties could make computers run a million times faster and store information with a million times more energy efficiency. | |
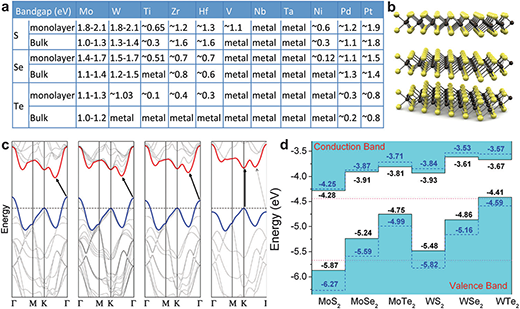 |
|
| Two-dimensional transition metal dichalcogenides (2D-TMDs). (a) The table shows common TMDs and their band gap. (b) A schematic illustration of the layered structure of MoS2. (c) Energy dispersion in bulk, quadrilayer (4L), bilayer (2L) and monolayer (1L) MoS2 from left to right. The horizontal dashed line represents the energy of a band maximum at the K point. The red and blue lines represent the conduction and valence band edges, respectively. The lowest energy transition increases with the decreasing layer and evolve from indirect to direct (vertical) transitions. (d) The relative valence and conduction band edge of some common TMDs (monolayer).(© The Royal Society of Chemistry) (click on image to enlarge) | |
Electronic and Optoelectronic Devices Based on 2D TMDs |
|
| Early research in 2D-TMDs has focused on their potential as a new generation of atomically thin semiconductors for functional electronics and optoelectronics. In 2013, researchers demonstrated the first n-type field-effect-transistor (FET) made of a monolayer of tungsten diselenide (WSe2), showcasing the material's potential for future low-power and high-performance integrated circuits. | |
| With intrinsic bandgaps typically in the 1-2 eV range, 2D-TMDs overcome graphene's key shortcomings for electronic applications, making them ideal for use in transistors. Additionally, TMDs have excellent absorption properties for circularly polarized light, making them suitable for use in detectors. | |
Synthesis of Atomically Thin 2D Transition Metal Dichalcogenides |
|
| Initial TMD research and device demonstrations often relied on exfoliated flakes, which limited the flake size to around 10 µm or less. This led to the fabrication of 2D-TMD heterostructure devices through labor-intensive exfoliation and repeated physical transfer processes, an approach not scalable for practical technologies. | |
| To fully explore TMD potential, researchers have developed two distinct synthetic strategies: top-down approaches, including mechanical, chemical, and solvent exfoliation; and bottom-up strategies, which involve chemical synthesis of atomically thin nanosheets in solution phase or through chemical vapor deposition processes. | |
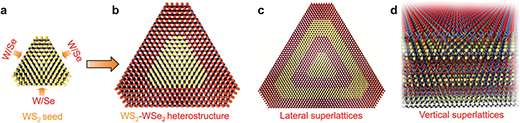 |
|
| Growth of 2D-TMD heterostructures. (a and b) A schematic illustration of lateral epitaxial growth of WS2–WSe2 heterostructures. (c) A schematic illustration of successive lateral epitaxial growth of superlattice structure in the lateral dimension. (d) A schematic illustration of van der Waals superlattices through the successive layer-by-layer growth approach. (© The Royal Society of Chemistry) (click on image to enlarge) | |
Conclusion |
|
| Although the field of TMD research is still in its early stages, the versatility and unique properties of these materials hold great promise for the future of advanced technologies. The availability of a wide range of 2D-TMDs with variable electronic band structures enables the creation of diverse heterojunctions and superlattices with designed and optimized band alignment. By precisely tuning the number of atomic layers and electronic functions, researchers can create a powerful material platform for novel, high-performance electronic and optoelectronic devices. | |
| As research progresses and scalable synthesis methods are refined, the potential applications for TMDs will continue to expand. These developments could lead to significant advances in fields such as energy storage, solar cells, sensors, and wearable electronics. The unique properties and potential applications of transition metal dichalcogenides make them an exciting and promising area of research in material science and nanotechnology. | |
| Fur further reading, we recommend this article in Nature Reviews Materials: "2D transition metal dichalcogenides". | |
Frequently Asked Questions (FAQs) about Transition Metal Dichalcogenides (TMDs)What are Transition Metal Dichalcogenides?
Transition Metal Dichalcogenides (TMDs) are a type of layered material, formed from one of the transition metals and either sulfur, selenium or tellurium - the chalcogens. The layer structure of these materials allows them to possess unique electronic, optical, and mechanical properties, which makes them interesting for a wide range of applications, from nanoelectronics to energy storage.
What are the key properties of Transition Metal Dichalcogenides?
TMDs are known for their semiconducting properties, high surface-to-volume ratio, strong light-matter interaction, and mechanical flexibility. The electrical and optical properties can also be modified by changing the number of layers, making them versatile materials for device engineering.
What applications do Transition Metal Dichalcogenides have?
TMDs are being studied for use in a variety of fields, including electronics, optoelectronics, energy storage, catalysis, and sensors. Their high surface-to-volume ratio makes them excellent for applications in catalysis and energy storage, while their tunable bandgap and high carrier mobility make them suitable for electronics and optoelectronics.
How are Transition Metal Dichalcogenides synthesized?
There are several methods to synthesize TMDs, including chemical vapor deposition (CVD), physical vapor deposition (PVD), and atomic layer deposition (ALD). The choice of method depends on the desired properties of the final product, such as thickness, layer uniformity, and crystalline quality.
What challenges exist in working with Transition Metal Dichalcogenides?
TMDs can be difficult to synthesize in a controlled manner, with uniform layers and high crystal quality. In addition, they can be sensitive to environmental conditions, such as oxygen and moisture, which can degrade their properties. This makes it challenging to manufacture and use them in a practical, reliable way.
How do Transition Metal Dichalcogenides differ from graphene?
While both TMDs and graphene are layered materials, they have some key differences. Graphene is a zero-bandgap semiconductor, while TMDs have a tunable bandgap, which makes them suitable for applications in electronics and optoelectronics. In addition, while graphene has superior carrier mobility, TMDs offer better light-matter interaction and a high surface-to-volume ratio.
What is the environmental impact of Transition Metal Dichalcogenides?
The environmental impact of TMDs largely depends on the methods used to synthesize and process them. Some methods can involve hazardous chemicals and generate waste. However, they are also being studied for use in green technologies, such as solar cells and energy storage systems.
How do the properties of Transition Metal Dichalcogenides change with different transition metals?
The properties of TMDs can vary significantly depending on the transition metal used. For example, molybdenum disulfide (MoS2) and tungsten diselenide (WSe2) have different bandgaps, carrier mobilities, and light absorption characteristics, which can be exploited for different applications.
What is the future of Transition Metal Dichalcogenides in electronics?
TMDs have the potential to revolutionize the electronics industry due to their unique properties, such as tunable bandgap and high carrier mobility. They could enable the development of new devices, such as flexible electronics, high-performance transistors, and photodetectors. However, challenges in synthesis and fabrication still need to be overcome.
How can Transition Metal Dichalcogenides be used in energy storage?
TMDs' high surface-to-volume ratio and tunable electronic properties make them promising materials for energy storage applications, including batteries and supercapacitors. Their layered structure allows for efficient charge transport and high storage capacity.
|
