| Jan 10, 2022 | |
Nanotechnology strategies for plant genetic engineering |
|
| (Nanowerk Spotlight) Humanity's efforts to modify food plants is as old as farming itself, some 10,000 years. Before genetic engineering became possible, farmers have used simple selection inter- and intraspecies and crossing – for instance, today's strawberries are a cross between a strawberry species native to North America and a strawberry species native to South America. As biotechnologies progressed, more advanced techniques like somatic hybridization (cell fusion), somaclonal variation and mutation breeding were developed. | |
| Genetic engineering began in 1973, when biochemists Herbert Boyer and Stanley Cohen developed genetic engineering by inserting DNA from one bacteria into another (read more about genetically modified organisms in this excellent primer by the FDA: Science and History of GMOs and Other Food Modification Processes). | |
| The term genetic engineering describes a process in which a type of genetic modification is made to an organism's genome that involves an intended targeted change in a plant or animal gene sequence to effect a specific result through the use of DNA and, more recently, RNA technology. | |
| Plant genetic engineering can rapidly and directionally improve the target traits of crops, break the bottleneck of conventional breeding, and achieve simultaneous improvement of crop quality and yield to meet human needs. | |
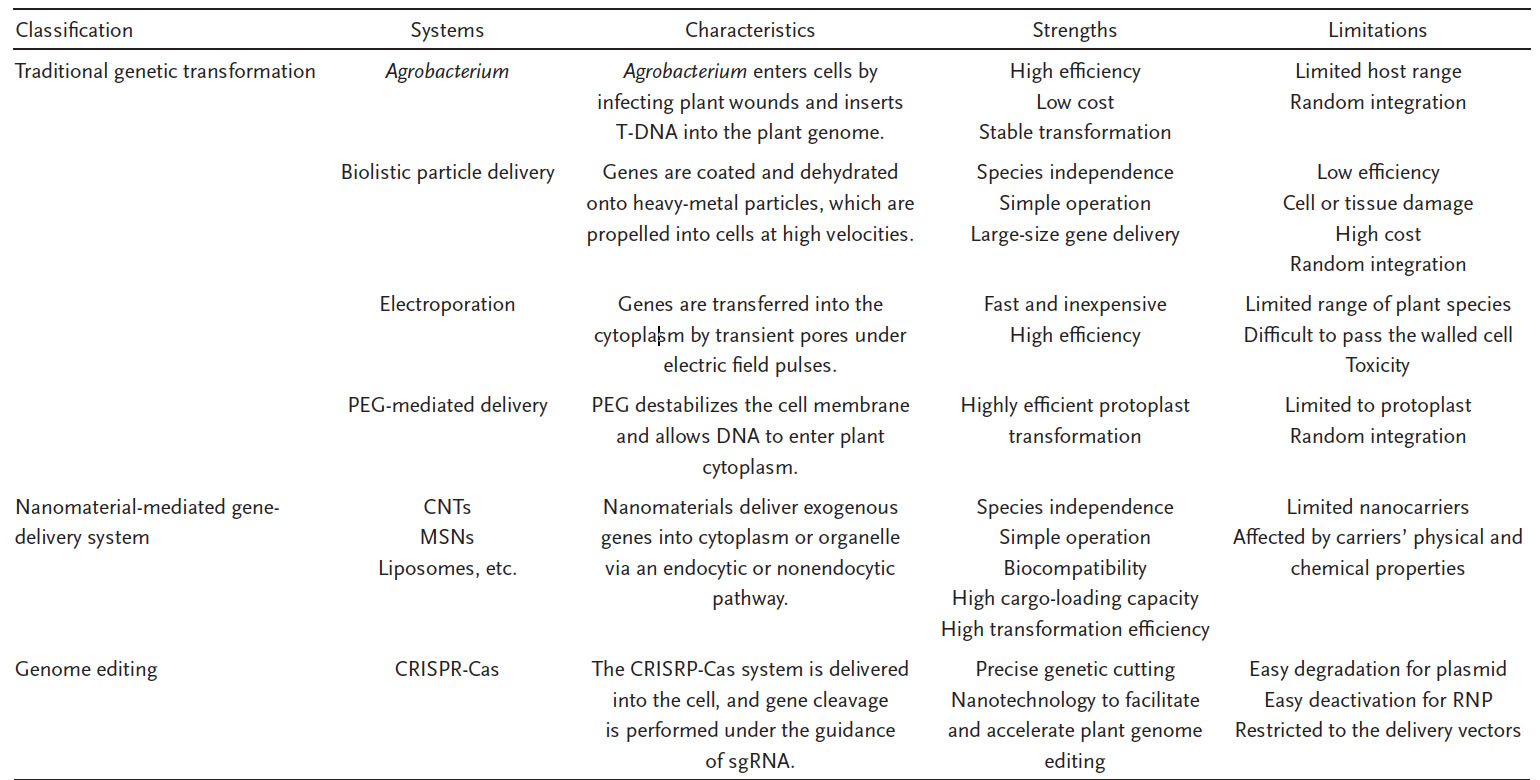
| Due to the rapid development of nanotechnology in the past few decades, nanomaterials are widely used in nanobiology and gene therapy due to their small size, large surface area, biocompatibility, biodegradability, low toxicity, and low-immunogenic properties. The table below summarizes the key differences, the strengths and the limitations of different genetic transformation techniques: | |
 |
|
| Comparison between various gene-delivery systems used for plant genetic engineering. Plant genetic engineering can be categorized into three main groups: traditional plant transformation, nanomaterial-mediated gene-delivery system, and genome editing. Each has its characteristics, strengths, and limitations. Abbreviations: T-DNA, transfer DNA; CNTs, carbon nanotubes; MSNs, mesoporous silica nanoparticles; CRISPR-Cas, clustered regularly interspaced short palindromic repeats-CRISPR-associated proteins; sgRNA, single-guide RNA; RNP, ribonucleoprotein. (Reprinted with permission by Wiley-VCH Verlag) (click on image to enlarge) | |
| However, the development of plant genetic engineering lags behind the development of animal genetic engineering. Plant cells differ from animal cells in several aspects, a major one being that, in addition to the cell membrane, they possess a wall surrounding them to provide mechanical and structural support. The presence of this plant cell wall, which only allows biomolecules with a diameter less than 20 nm to pass through, is limiting the application of nanomaterials in genetic engineering-mediated crop improvement. | |
| In recent years, breakthroughs in nanotechnology for genetic engineering have provided more favorable tools for the genetic transformation of plants. For instance, researchers have developed a technique, which uses nanoparticles to deliver genes into the chloroplasts of plant cells, and works with many different plant species, including spinach and other vegetables. In another study, carbon nanotubes enabled delivery of functional genetic material without DNA integration in mature plants. | |
| Based on the characteristics of nanomaterials, a new review article ("Nanotechnology Strategies for Plant Genetic Engineering") by scientists at the School of Food and Biological Engineering at the Hefei University of Technology, summarizes the types of gene carriers used in plant genetic transformation, the ways of combining with foreign genes, and the differences and advantages compared with earlier traditional transgenic methods. | |
| The authors also discuss challenges and perspectives of nanomaterial-mediated gene-delivery systems to provide new ideas for further optimizing the design and developing novel plant genetic transformation technologies. | |
 |
|
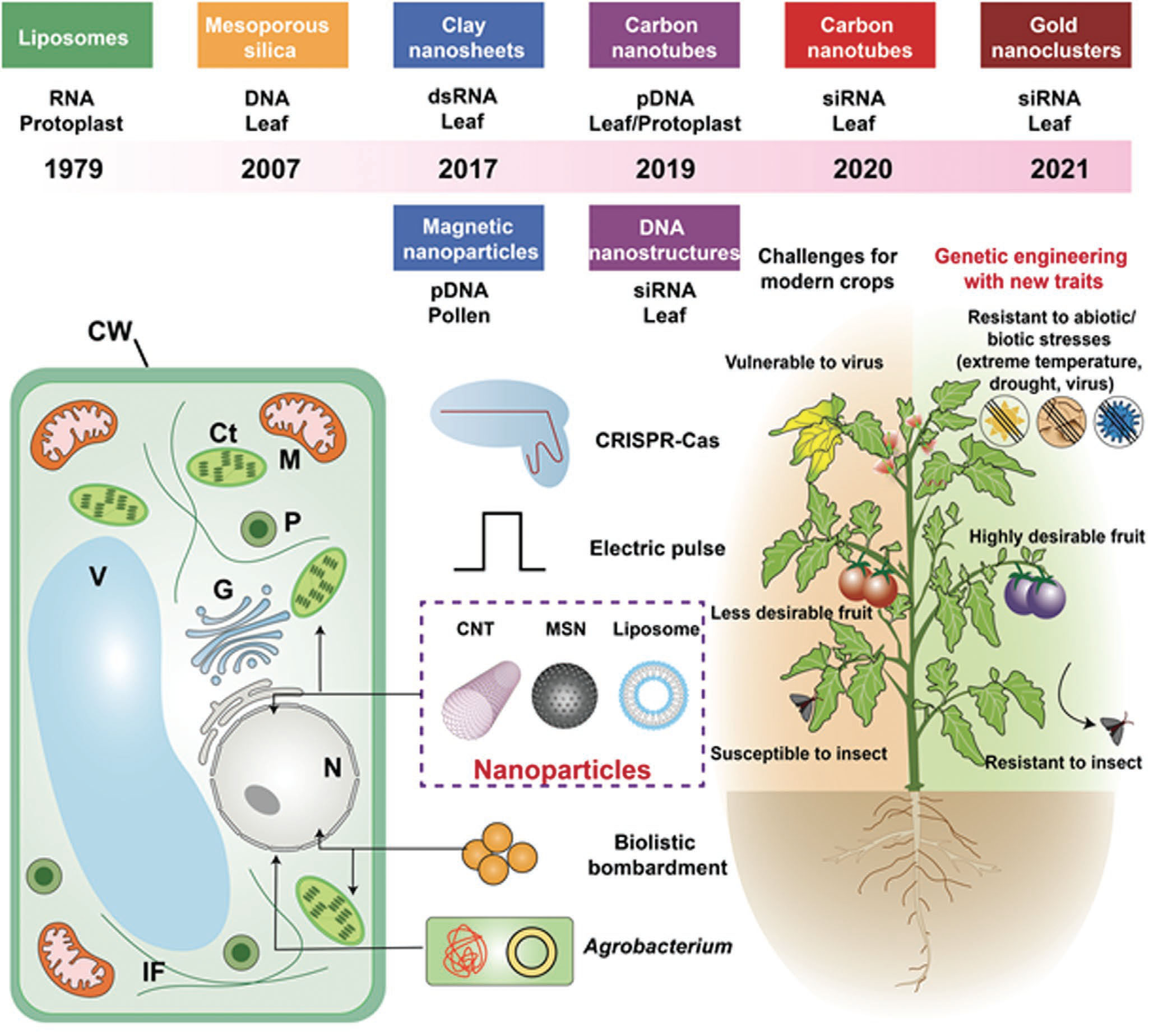
| Schematic showing nanomaterials developed for plant genetic engineering. In 1979, liposome nanoparticles as vectors were first demonstrated to mediate bacterial RNA uptake into carrot protoplasts by Matthews et al. In 2007, the first nanostrategy for intact plant genetic engineering was explored by Torney et al., where gene-loaded mesoporous silica was bombarded into plant leaves. In 2017, clay nanosheets were used to protect double-stranded RNA (dsRNA) and increase plant virus resistance, while magnetic nanoparticles realized transgenic plant construction by pollen magnetofection in the same year. Then, species-independent, high-efficient gene-delivery systems without physicochemical assistance were developed using carbon nanomaterials, DNA nanostructures, and gold clusters in the last three years. Physical (biolistic bombardment, electric pulse), chemical (poly(ethylene glycol) (PEG), not shown), or biological (Agrobacterium, CRISPR-Cas) approaches are extensively used in plant cells and whole plants. Nanomaterial-based gene-delivery method has been developed and advanced as the new gene-delivery system for plants. Plant genetic engineering confers crops with enhanced resistance to abiotic/biotic stresses (e.g., extreme temperature, drought, viruses, insects) and improved crop yield and quality. CW, cell wall; Ct, chloroplast; M, mitochondrion; G, Golgi apparatus; N, nucleus; V, vacuole; P, peroxisome; IF, intermediate filaments. (Reprinted with permission by Wiley-VCH Verlag) (click on image to enlarge) | |
| Compared to the traditional plant transformation methods, nanomaterial-mediated plant genetic transformation has the following advantages: it can pass through the plant cell wall passively without a power device; it can achieve large fragments of genes and multigenetic transformation by loading a large number of nucleic acids; it has no host restriction; it can protect exogenous nucleic acids, reduce the degradation rate of nucleic acids in cells and improve genetic transformation efficiency. | |
| Most studies have suggested that the internalization efficiency of nanoparticles was positively correlated with genes being delivered. Most of these particles – such as quantum dots, nanoparticles, nanotubes, liposomes, DNA nanostructures – need to be less than 20 nm in at least one dimension. In addition, smaller nanomaterials have unique advantages which can achieve suborganelle localization (e.g., chloroplast, mitochondrion, nucleus). The use of various nanomaterials like carbon nanotubes, magnetic nanoparticles, and mesoporous silica nanoparticles for nucleic acid delivery in plant cells has been reported as proof of concept. | |
| So far, most scientific research is focused on the interactions between carbon nanomaterials and mammalian cells. Research on whether and why carbon nanomaterials can be exploited as carriers to deliver foreign genes into plant cells is still in its early stages. | |
| CRISPR-Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats-associated protein 9) is considered a 'molecular scissors' technique that can be used for precision plant breeding by deleting, replacing, or editing the nucleic acid sequence. Recently, nanoparticle-based CRISPR-Cas delivery has shown advantages over traditional methods, including improved stability, low toxicity, high loading capacity, wide recipient plant species, etc ("Nanotechnology-based delivery of CRISPR/Cas9 for cancer treatment"). For example, liposomes could be designed to encapsulate CRISPR-Cas systems in several forms (e.g., cationic liposomes; multilamellar neutral liposomes). Other nanomaterials, such as gold nanoparticles, also found their ways in delivering CRISPR-Cas systems in medical applications. However, whether nanoparticles can mediate CRISPR-Cas genome editing in plant cells or organelles is still unknown. | |
 |
|
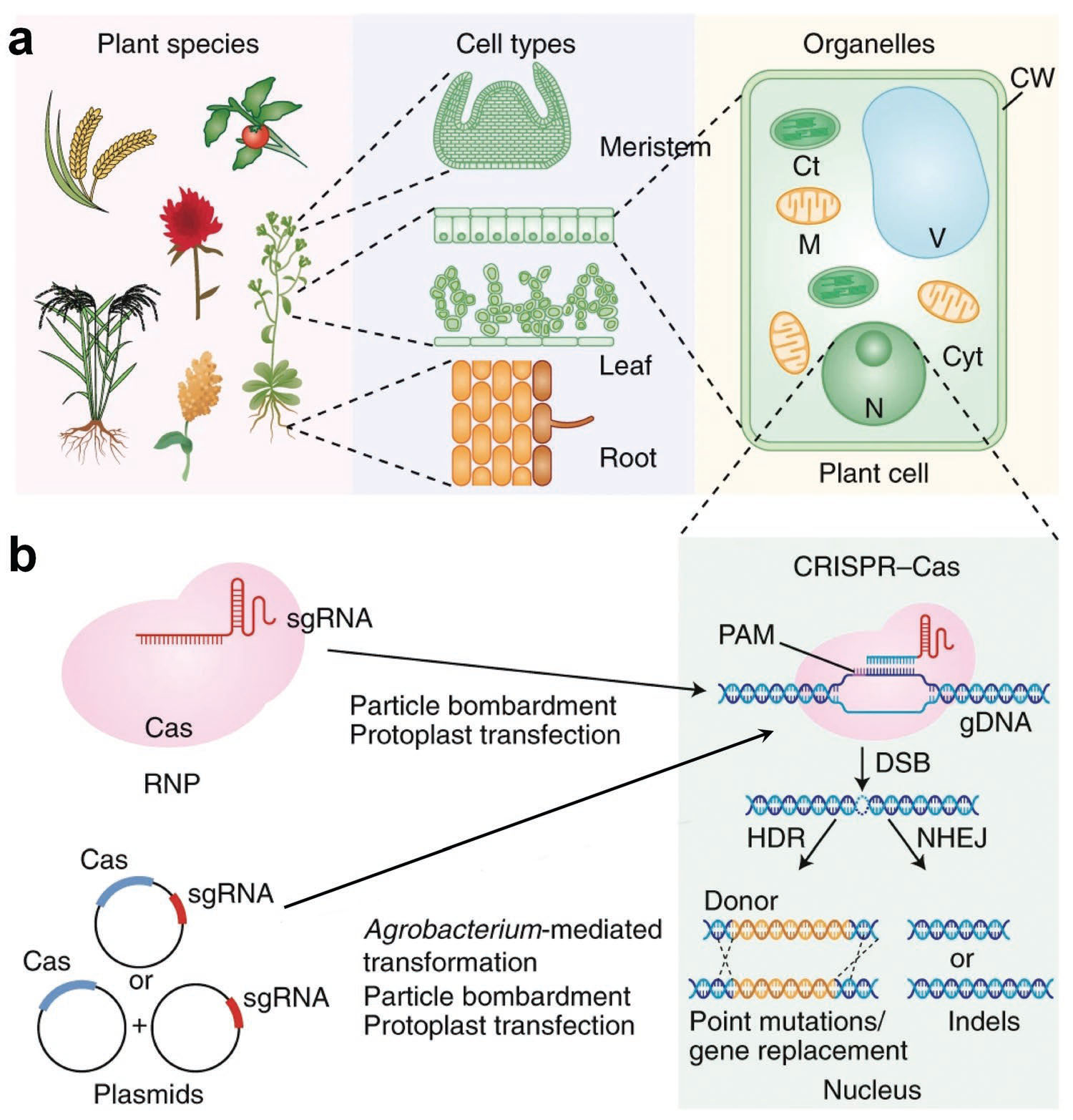
| Delivery of CRISPR-Cas reagent to diverse plant species, cells, and organelles. a) Examples of plant species, cell types, and organelles that the CRISPR-Cas system can target. b) CRISPR-Cas elements can be delivered into the cells to perform genome editing. The Cas protein cleaves the DNA with the help of sgRNA. Once the double-stranded break (DSB) is generated, DNA repair mechanisms are triggered: HDR and NHEJ. Ct, chloroplast; Cyt, cytoplasm; CW, cell wall; sgRNA, single-guide RNA; gDNA, genomic DNA; M, mitochondrion; N, nucleus; V, vacuole. (Reprinted with permission by Wiley-VCH Verlag) | |
| Concluding their review, the authors note that nanotechnology for plant genetic engineering is a novel emerging field where still many problems need to be solved; in particular: | |
| "First, how are nanomaterials internalized into plant cells without external assistance? This issue needs to be further clarified. Previous studies suggested that the size, shape, aspect ratio, tensile strength, compactness, colloid stability, and electric charge of nanomaterials directly influenced their transport mechanisms in plant cells. These factors could realize collaborative internalization. Based on systemically analyzing plant cell transport mechanisms of nanomaterials, we can design and synthesize appropriate nanocarriers through establishing the mathematical model of nanomaterials??? transport process in plant cells. | |
| "Second, is the internalization of nanomaterials necessary for plant gene delivery? Zhang et al. found that gold nanospheres having difficulty entering plant cells were still effective for delivering nucleic acids. The underlying mechanism needs to be further explored. | |
| "Thirdly, can nanomaterials effectively deliver CRISPR-Cas elements into plants? CRISPR-Cas enables precise genome editing, while it can be easily degraded (for DNA) or inactivated (for Cas protein) within plants. The use of nanomaterials to achieve organelle targeted delivery will further promote the development of plant genetic engineering. | |
| "Additionally, the cytotoxicity of nanomaterials needs further study." | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
