| Oct 11, 2023 | |
Preventing medical implant infections with nitric oxide generating MOF coatings |
|
| (Nanowerk Spotlight) Medical implants from artificial joints to pacemakers improve quality of life for millions. However, bacteria can colonize the surface of these devices, forming resilient biofilms and sparking stubborn infections. Around half of orthopedic implants become infected within the first five years, often necessitating repeated surgery or implant removal. | |
| Preventing bacterial adhesion is key to avoiding these complications. Implant coatings that slowly release nitric oxide could help deter biofilm formation through their potent antimicrobial properties. Nitric oxide inhibits bacterial growth, disperses existing biofilms, and helps the immune system combat infection. | |
| Unfortunately, current nitric oxide-releasing coatings have major limitations. Most rely on chemical breakdown of nitric oxide donor compounds embedded in the coating. This only provides short-term nitric oxide release as the donor depletes over time. | |
| Once the donor is fully consumed after a few weeks, the coating loses all antimicrobial activity. Bacteria can then colonize the implant surface, seeding an infection. Achieving sustained, on-demand nitric oxide generation has proven difficult. | |
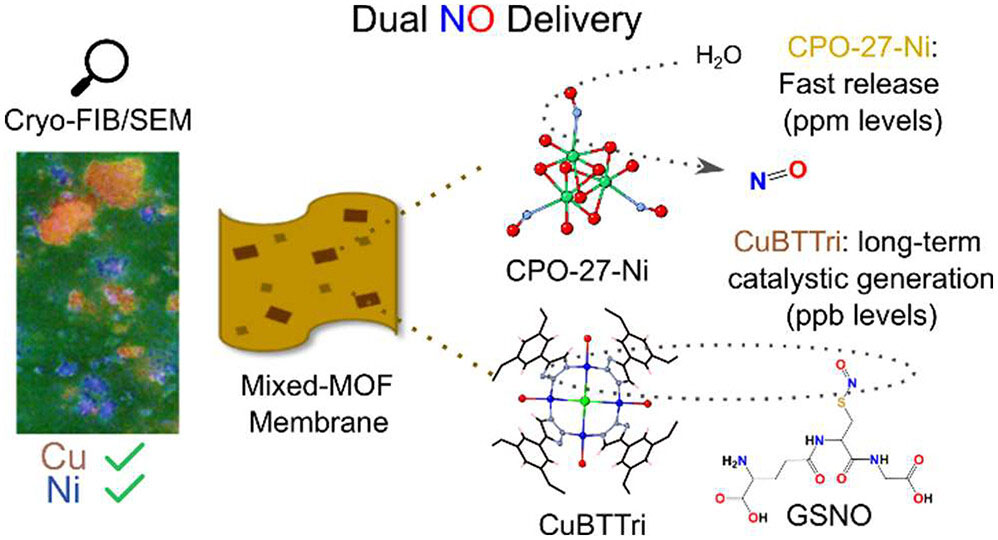
| To address this problem, researchers developed a new type of coating using metal-organic frameworks (MOFs) – highly porous materials made by linking metal ions with organic molecules. By incorporating two different MOFs into an implant coating, the team created a smart material capable of both rapid nitric oxide release and low-level sustained production. | |
 |
|
| Graphical abstract of the work. (© ACS) | |
| This twin threat approach combines an immediate bacteria kill with constant prevention of recolonization. In a study published in ACS Applied Materials & Interfaces ("Mixed Metal−Organic Framework Mixed-Matrix Membranes: Insights into Simultaneous Moisture-Triggered and Catalytic Delivery of Nitric Oxide using Cryo-scanning Electron Microscopy"), the researchers demonstrated the potential of these coatings to tackle implant infections. | |
| The team focused on two MOFs with unique methods of generating nitric oxide – CPO-27-Ni and CuBTTri. CPO-27-Ni stores nitric oxide within its porous structure which is displaced when water molecules enter the material. This provides a powerful nitric oxide burst ideal for initial bacterial eradication. | |
| Meanwhile, CuBTTri leverages the body's own nitric oxide resources. It catalyzes the conversion of S-nitrosoglutathione (GSNO) into nitric oxide. GSNO is an endogenous human metabolite, meaning CuBTTri can generate nitric oxide for extended periods by tapping into this biological reservoir. | |
| Integrating both MOFs into a single coating imparts rapid bacteria kill thanks to CPO-27-Ni along with sustained biofilm prevention due to the steady nitric oxide production from CuBTTri. | |
| To fabricate the dual-MOF composites, the researchers dispersed the MOF particles into a medical-grade polyurethane film. By tweaking the ratio of CPO-27-Ni and CuBTTri added, they tuned the nitric oxide release kinetics. | |
| The team produced films loaded with only CPO-27-Ni, only CuBTTri, or a 50/50 combination. Analyzing the distribution of the microscopic MOF particles within the coatings proved challenging using conventional microscopic techniques. | |
| Instead, the researchers utilized cryogenic scanning electron microscopy (cryo-SEM). Freezing the samples prevented damage during imaging while achieving nanoscale resolution. This revealed both MOFs were thoroughly dispersed throughout the polymer matrix. | |
| Measurements confirmed the coatings performed as designed. The CPO-27-Ni films displayed rapid nitric oxide discharge upon exposure to moisture, while only the CuBTTri films catalyzed slow but sustained nitric oxide production in the presence of GSNO. | |
| Excitingly, films containing both MOFs successfully combined an initial high-level nitric oxide surge with subsequent low-level generation. This dual activity could help deter implant infections through both immediate bacteria killing and constant prevention of regrowth and recolonization. | |
| Beyond implants, these coatings could be applied to wound dressings, intravenous catheters, and other medical devices vulnerable to bacterial fouling. With further development, multi-MOF composites may even permit on-demand delivery of other therapeutic gases like hydrogen sulfide. | |
| This research highlights the potential of MOFs to transform passive materials into intelligent surfaces with unprecedented control over drug delivery, gas transport, and sensing. Cryo-SEM imaging provides an important tool to advance multi-MOF composites and other complex materials into real-world applications. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
