| Nov 23, 2023 | |
New nanotechnology makes water harvesting from air far easier |
|
| (Nanowerk Spotlight) For over half the global population, accessing clean drinking water remains an enduring challenge. With climate change and development pressures exacerbating water scarcity worldwide, innovative solutions are desperately needed. Atmospheric water harvesting – the extraction of drinkable water directly from humid air – offers immense promise if made efficient and affordable enough for mass deployment. | |
| Previous water harvesting technologies have faced major limitations. Materials like zeolites and silica gel adsorbents offer insufficient capacity, slow kinetics and high regeneration temperatures above 100 °C. Metal-organic frameworks (MOFs) boast excellent uptake even in dry conditions but suffer poor stability from hydrolysis reactions degrading their metal-organic bonds. Super moisture absorbent hydrogels are cheap but kinetics too sluggish for continuous operation. | |
| The most attractive option may be hygroscopic salts which avidly soak up water vapor through deliquescence. However, their tendency to uncontrollably dissolve away makes containment and reuse difficult. Confining salts in porous matrices helps but remains prone to leakage over operating cycles. No current system combines high uptake speeds, capacities across a wide humidity range, low temperature release and durable long-term stability. | |
| Advanced functional nanomaterials may finally help atmospheric water harvesting become truly viable and make a global impact. As reported in a recent paper in Small ("Efficient Atmospheric Water Harvesting of Superhydrophilic Photothermic Nanocapsule"), researchers at Beihang University in Beijing have created an innovative ‘superhydrophilic photothermic hollow nanocapsule’ (SPHN) that overcomes multiple limitations facing current technologies. | |
| Their testing demonstrates rapid adsorption even in dry conditions, huge storage capacities exceeding the material’s own weight in water, swift low-temperature solar-driven release of nearly all adsorbed liquid, and exceptional cycling endurance over repeated use. | |
 |
|
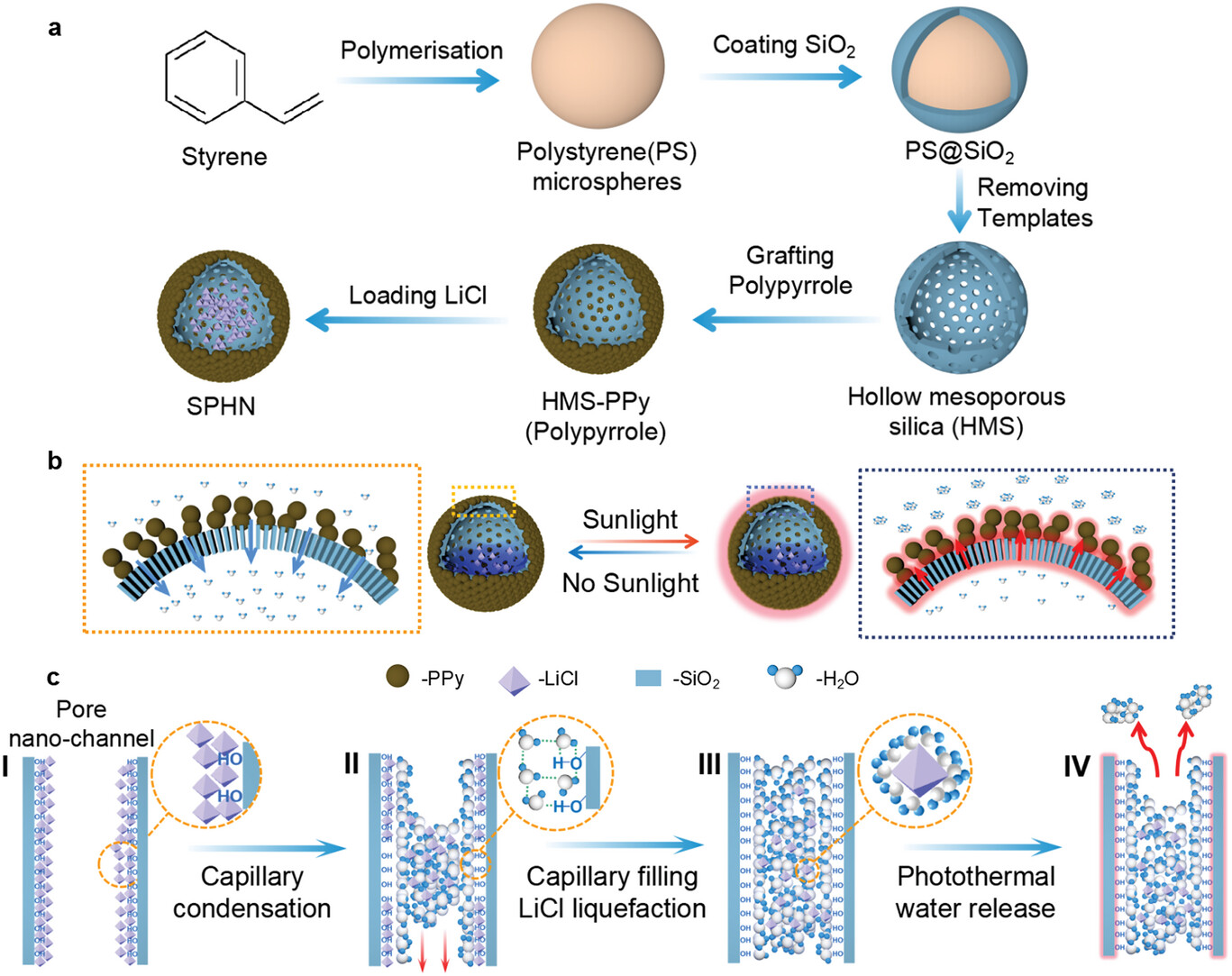
| Illustration of the synthesis and micromechanism of superhydrophilic photothermic hollow nanocapsules (SPHN). a) Synthesis of SPHN. Polystyrene (PS) of a specific size is prepared by a polymerization reaction, a layer of SiO2 is coated on the PS surface to obtain PS@SiO2, and the PS template is thermally removed to obtain HMS. The photothermal layer of PPy is grown on the surface of HMS to obtain HMS-PPy. Then the HMS-PPy is immersed in 37 wt% LiCl solution and loaded with LiCl. SPHN is successfully achieved. b) Diagram on AWH of SPHN. In no sunlight, water molecules can be collected from the air and stored in the cavity (left inset, see blue arrows). In sunlight, the water in the cavity is released in the form of molecular clusters (right inset, see red arrows). c) Nano-channel role in SPHN for AWH. In nano-channel, small amounts of LiCl are present there (Frame I). In no sunlight, water molecules from the air contact with nano-channel of SPHN. By capillary condensation of water molecules (Frame II), the solution of LiCl hydrates forms, and is transported to the cavity of SPHN for storage (see red down-arrows in Frame II). As the hygroscopic LiCl liquefies to fill the nano-channels, the LiCl solution continues to capture water from the air until saturation (Frame III). In sunlight, PPy converts the sunlight energy into heat to act the LiCl solution. Water molecular clusters in the nano-channels is released (Frame IV, see red top-arrows) that can be replenished over time by capillary forces, reducing the heat of water desorption for rapid water release under photothermal action. (Reprinted with permission by Wiley-VCH Verlag) | |
| This breakthrough leverages recent advances in precision multi-component nanofabrication to seamlessly combine the strengths of several promising approaches within a novel hierarchical architecture. At the heart lies nanoporous hollow silica spheres, whose hollow interior provides a protected reservoir for absorbed water. Grafting the external surface with light-absorbing polypyrrole polymer gives the material strong photothermal properties to heat its contents when exposed to sunlight. Finally, the chemically hydrophilic interior is infused with hygroscopic lithium chloride salt to strongly pull in atmospheric moisture. | |
| The nanocapsules’ tiny size and porous shell maximize surface contact area for ultrafast vapor adsorption while weak surface interactions with water molecules reduce the energy needed to release them as a liquid. Liquefied salt is wicked into the hollow interior through capillary action within the nanopores, avoiding external spillage. This stored hypersaline solution heats up readily when the sunlight-absorbing polymer coat is irradiated, forcing out captured water which instantly vaporizes in the hot, dry external environment. Rising vapor then condenses on a cool surface and drips into a collection tank for later use, closing the extraction loop. | |
| Testing demonstrated exceptional performance meeting or exceeding all key benchmarks for an ideal water harvesting medium. Adsorption speed is rapid, with over 75% of maximum capacity reached within an hour even in bone-dry 30% relative humidity air. Total uptake capacity scales linearly with humidity, soaking up 78%, 140% and 200% of the nanocapsules’ own weight at 30%, 60% and 80% RH respectively. This far outstrips conventional zeolites or silica gel, while avoiding stability issues facing MOFs and slow absorption kinetics of hydrogels. | |
| Photothermal heating efficiency is similarly remarkable. Under just 0.7 to 1 kW per m2 solar flux akin to natural direct sunshine, the nanocapsules swiftly reach over 75 °C to drive off nearly all stored moisture content as evaporable vapor within an hour. Critically, even much lower 0.4 kW per m2 irradiation still liberates half the absorbed liquid, enabling solar-powered regeneration even on cloudier days. | |
| Furthermore, absorption-desorption cycling shows excellent reproducibility over repeated water harvesting use without performance declines, backed up by rigorous humidity exposure tests indicating no salt leakage or agglomeration issues. This demonstrates the hollow nanocapsules successfully contain deliquesced salt over long-term operation. Outdoor condenser trials harvested up to 1.2 grams of water per gram of material over daily solar-powered cycles. Collected condensate met WHO drinking standards for critical health metrics. | |
 |
|
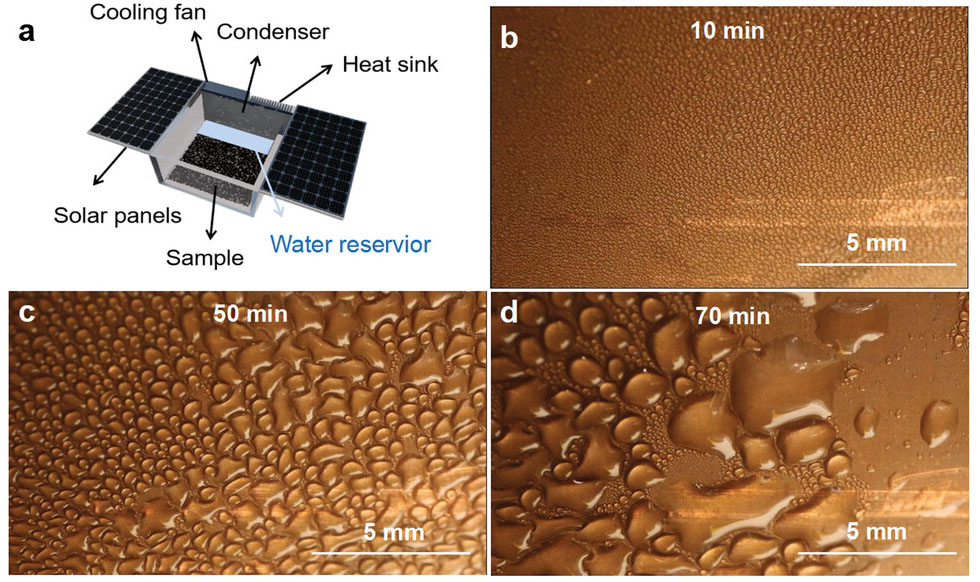
| Photographs of water harvesting as SPHN in outdoor. a) Illustration of AWH device. It is composed of a condenser, a chamber, and two solar panels. b–d) Photograph of continuous outdoor water harvesting with as-recorded time. b) At 10 min, much more small water droplets cover the condensing sheet. c) At 50 min, the larger water droplets distinctly appear. d) At 70 min, the coalesced-droplets become more larger and shed off, which is then collected. (Reprinted with permission by Wiley-VCH Verlag) | |
| The nanocapsules’ combination of strong environmental response, intense photothermal output, speedy adsorption/desorption kinetics, huge uptake capacity and cycling durability offers major advantages over existing atmospheric water harvesting technologies. Their performance metrics either meet or significantly exceed recently reported alternatives across the board while avoiding issues like limited humidity range or chemical instability. Notably, the adsorbent structure is readily manufacturable at scale through established template synthesis processes. | |
| With further development, the approach could enable self-powered atmospheric water generators as standalone devices or modular add-ons for structures like homes, greenhouses or industrial plants. Units sized for a single family’s daily drinking and cooking needs up to community-level supply are envisaged. Solar-regenerated operation without grid electricity makes systems deployable even in unelectrified rural districts and urban slums lacking water infrastructure. | |
| The researchers suggest such atmospheric water harvesters could sustainably supply clean water to remote settlements, disaster relief camps, off-grid farms or households reliant on distant contaminated sources. Even households already piped to centralized water grids may benefit from small backup generators offering resilience against supply failures during droughts or heat waves which climate change will exacerbate. | |
| If costs become sufficiently low, adoption in water-stressed urban and agricultural sectors could significantly relieve pressure on stressed natural waterbodies, aquifers and reservoirs suffering worrisome drawdown in regions like the American West, Southern Europe, Northern Africa, the Middle East, India, China and Australia. Atmospheric water harvesting at scale may even emerge as a supplemental source for cities’ existing water infrastructure. | |
| With intensifying freshwater scarcity widely seen as an existential near-future threat, the breakthrough’s enormous promise has attracted intense interest. Licensing is already underway exploring applications from emergency relief to electric utility cooling systems. Commentators suggest the innovative nanotechnology could make a transformative impact mitigating global water risks and improving quality of life if uptake spreads widely. The researchers plan to further optimize efficiency and pilot larger prototype devices over the coming years in partnership with several major manufacturers. | |
| If the technology’s potential is fulfilled, every building could one day harvest its own water supply from thin air instead of tapping distant lakes and aquifers. The approach might help curb severe scarcity for nearly half Earth’s population, securing a critical resource threatened by climate change and development. What once sounded like science fiction may soon offer society a desperately needed adaptation strategy as the hotter, drier century unfolds. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
