| Jan 08, 2024 | |
Bacteriophage-powered micromotors for rapid and selective Point-of-Care bacteria detection (w/video) |
|
| (Nanowerk Spotlight) Misdiagnosis and delayed treatment of bacterial infections have long challenged medicine, contributing to antibiotic resistance and poor patient outcomes. Clinicians lack rapid, selective diagnostic tests to target narrow-spectrum antibiotics early in illness. Instead, imprecise broad-spectrum regimens remain routine despite fueling resistance. Recent advances offer new possibilities, yet inherent technical constraints have continued to limit feasibility. | |
| Now, Spanish scientists report an inventive micromotor approach that sidesteps past barriers through engineering at the nano and bio scale. In a paper published in Advanced Functional Materials ("Magnetic Bacteriophage-Engineered Janus Micromotors for Selective Bacteria Capture and Detection"), scientists from the Universidad de Alcalá describe tiny hybrid machines called “bacteriophage micromotors” that efficiently detect Escherichia coli bacteria down to medically relevant concentrations. | |
| These self-propelling microscopic particles use tailor-made viruses to recognize specific bacteria strains. The viruses bind to matching bacteria to form unique sandwich structures that are then labeled for easy visual identification. Critically, the viruses only attach to their intended targets, conferring high selectivity amidst complex clinical samples. | |
 |
|
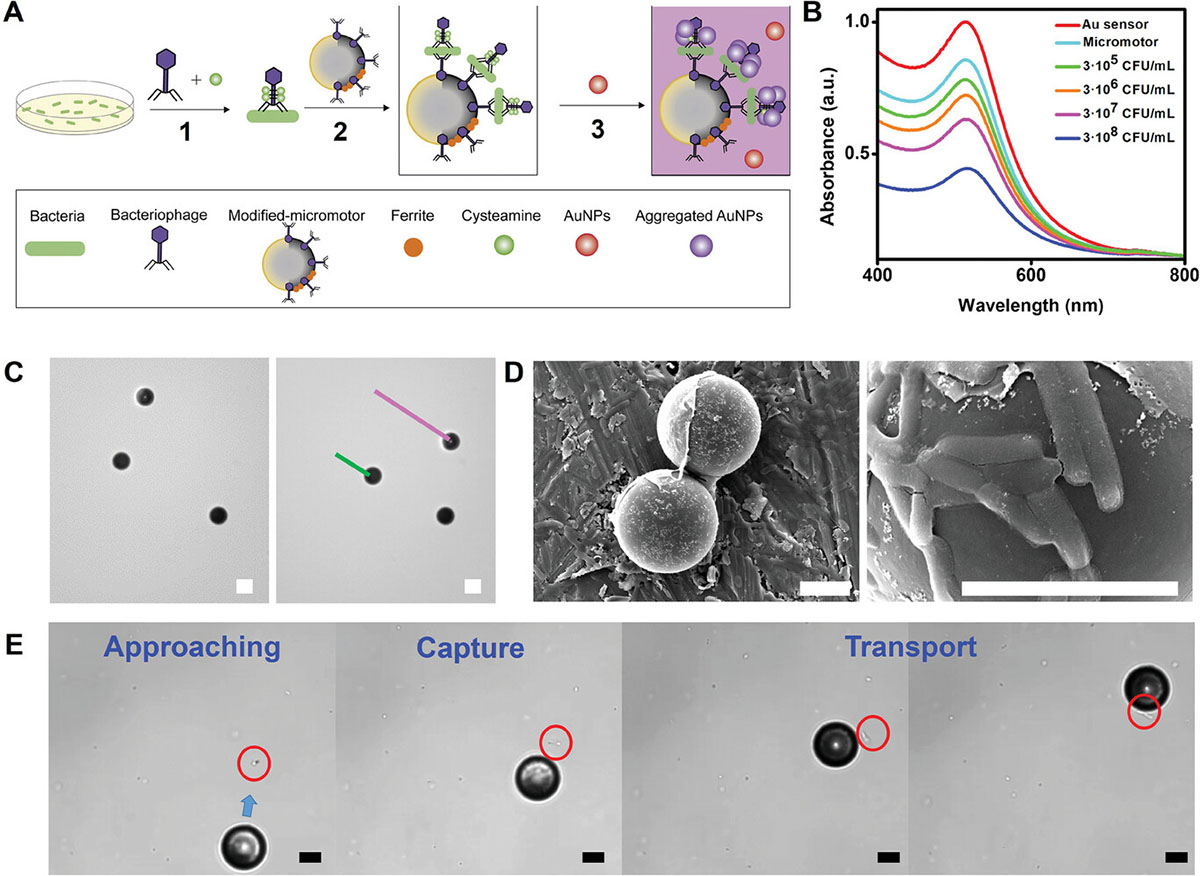
| A) Schematic of the bacteriophage-functionalized magnetic Janus micromotors for E. coli bacteria biosensing: 1) Generation of the bacteriacysteamine T4 bacteriophage complex. 2) Incubation of the bacteria-T4 bacteriophage complex with the magnetic phage-modified micromotors. 3) On-the-fly bacteria capture with the micromotors and addition of the AuNPs. The assay was performed in 96 well plates, following measurements with a microplate reader. B) UV–vis spectra of the AuNPs added to the incubated micromotors, with increasing bacteria concentration. C) Time-lapse images illustrating the magnetic motion of the micromotors and corresponding tracking lines. D) Scanning electron microscopy (SEM) images of the micromotors after bacteria capture at two magnifications. E) Time-lapse images showing a micromotor approaching, capturing, and transporting an E. coli bacterium. Scale bars, 10 µm. (Reprinted with permission by Wiley-VCH Verlag) | |
| The bacteriophage micromotors build on recent advances in asymmetric particulate design that impart distinct functions to different hemispheres of individual particles. This study used 20 micrometer polystyrene spheres coated on one side with iron oxide nanocrystals to enable directional magnetic propulsion and graphene oxide on the other to anchor genetically modified bacteriophages. By incorporating both motion and sensing functions into single integrated Janus units, the micromotors can actively mix tiny sample volumes by propelling at speeds up to 40 micrometers per second to increase sensor-target interactions. | |
| Key to the approach are the engineered bacteriophages that recognize specific bacteria subtypes. The T4 virus chosen in this study precisely identifies E. coli “strain B” via particular sugars on the bacterial membrane absent in other cells. This specificity addressed a primary limitation of past micromotor biosensing attempts that employed more generic lectin or carbohydrate baits prone to misleading false positives. | |
| The researchers demonstrated the technique by adding bacteriophage-micromotors to solutions of E. coli "strain B" bacteria tagged with complementary T4 viruses. The self-propelling particles then converged on and captured matching cells to indicate their presence through straightforward colorimetric assays. | |
| Quantifying aggregated gold nanoparticle absorption on standard 96-well plates enabled bacteria detection down to 100,000 colony forming units per milliliter – sufficient sensitivity for diagnosing urinary tract infections. Control trials with interfering Staphylococcus aureus bacteria or endogenously infected patient urine samples showed excellent selectivity. | |
| Beyond achieving speed, sensitivity, and specificity goals, the bacteriophage micromotor method uniquely integrates with existing clinical instrumentation. Avoiding sophisticated microscopes or complex analyses, the technology outputs easy yes-no colorimetric results standard plate readers can interpret. This practicality signals viability for mainstream adoption pending further validation. | |
| The researchers suggest their core approach could extend to sensing diverse pathogens by swapping tailored bacteriophages. Given the acute need for rapid bacteria detection tools, the innovative biologically empowered micromachine design seems poised to make meaningful practical impacts if successfully translated to human use. With infectious disease long plaguing medicine, hybrid bacteriophage-micromotors may finally offer clinicians the rapid and selective diagnostic capability needed to improve antibiotic stewardship and infection survival. | |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Become a Spotlight guest author! Join our large and growing group of guest contributors. Have you just published a scientific paper or have other exciting developments to share with the nanotechnology community? Here is how to publish on nanowerk.com. |
|
