| Posted: Jul 18, 2017 | |
Stimuli-responsive 'cluster bombs' for tumor therapy(Nanowerk News) Researchers have designed a a pH-responsive size-changeable “Cluster Bomb” based on mesoporous silica nanoparticle (MSN, ∼ 50nm) and peptide tLyP-1-modified tungsten disulfide quantum dots (WS2-HP) programmed tumor therapy. |
|
| By harnessing the rapid acid-responsiveness of benzoic-imine bond, the team from Wuhan University obtained a promising antitumor nanomedicine DOX@WS2-HP that is stable in systemic circulation and can break into electropositive DOX@MSN-NH2 and small-sized WS2-HP immediately after tumor accumulation. | |
| The findings have been reported in ACS Nano ("Stimuli-Responsive “Cluster Bomb” for Programmed Tumor Therapy"). | |
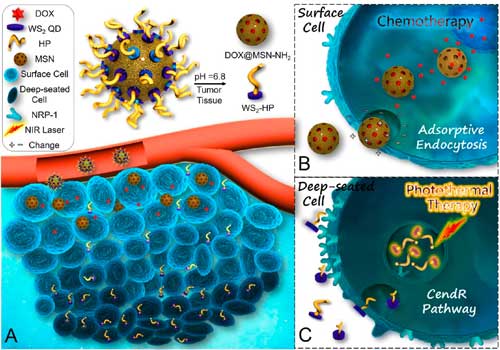 |
|
| (A) Once docking in the mild acidic tumor microenvironment, the acid-labile nanoplatform can rapidly disintegrate into electropositive DOX@MSN-NH2 and small-sized WS2-HP with improved tumor penetrating ability. (B) DOX@MSN-NH2 can be internalized via adsorptive endocytosis and mediate chemotherapy in the surface tumor cells. (C) WS2-HP can penetrate the tumor parenchyma via CendR pathway and accomplish NIR light-triggered photothermal ablation of the deep-seated malignant cells. (© ACS) (click on image to enlarge) | |
| Limited tumor penetration is the main hindrance for nanomedicines in solid tumor treatment, attributed to its disorganized vasculature, increased interstitial fluid pressure, and dense extracellular matrix in the solid tumor microenvironment. | |
| Nanomedicines usually infiltrated into no more than one or two cell layers away from the blood vessels and failed to penetrate the tumor parenchyma throughout. | |
| Interestingly, the team's decorated tLyP-1 peptide can not only homing to 4T1 tumors but also facilitate the tumor penetration of small-sized WS2-HP via a NRP-1dependent CendR pathway. | |
| Unlike previous conventional tumor-penetrating strategies, the researchers exploited a hybrid strategy that integrated size control and functional peptide modification to enhance the tumor penetration of WS2-HP. | |
| The deep-penetrated WS2-HP could exert predominant capability of light-to-heat conversion for photothermal therapy among the interior malignant cells which are distant from blood vessels. | |
| Meanwhile, electropositive DOX@MSN-NH2 can be readily endocytosed by the exterior tumor cells near the blood vessels and exhibit outstanding chemotherapeutic efficiency in supplement for photothermal therapy. | |
| "Looking forward, in consideration of heterogeneity of tumor cells, this “Cluster Bomb” strategy that using different therapeutic modalities to treat the tumor cells in different depths may provide a good idea for tailored multimode therapy," the authors conclude their report. |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
