| Oct 07, 2016 |
First intracellular X-ray crystal structure of a human protein complex
|
|
(Nanowerk News) The first three-dimensional (3D) structure of a human protein complex within intact mammalian cells has been obtained directly by A*STAR scientists (Nature Communications, "An in cellulo-derived structure of PAK4 in complex with its inhibitor Inka1"). It could provide new opportunities in structural biology, in developing cellular sensors and in validating anti-cancer drugs that target a specific protein.
|
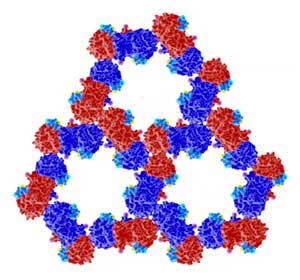 |
| The organization of proteins PAK4 (red and blue) and Inka1 (light blue) as determined from crystals formed inside cells. (Image: A*STAR Institute of Molecular and Cell Biology)
|
|
The complex generates a highly ordered honeycomb shape made of two proteins: PAK4 (p21-activated kinase 4) and its newly discovered inhibitor Inka1. PAK4 is essential for several cellular processes occurring in the junctions between cells, and when malfunctioning, plays a role in cancer metastasis.
|
|
“We discovered that Inka1 is the natural inhibitor of PAK4, and we observed by chance that when mammalian cells are engineered to make both these proteins, PAK4 spontaneously forms long crystals,” reveals Ed Manser, from the A*STAR Institute of Molecular Biology and Cell Biology. The group were the first to discover the PAK kinases.
|
|
Creating highly ordered arrangements of proteins, known as protein ‘crystals’, is the first step to determine their 3D structure through X-ray analysis. Since it is very rare for these crystals to form inside a cell, all protein 3D structures obtained so far have been crystallized artificially, from highly pure and homogeneous protein samples. In this study, however, the atomic structure can be determined without removing the crystals from the cells.
|
|
Teaming up with Robert Robinson's group, and using an X-ray microbeam to shoot at these tiny intracellular crystals, the scientists were able to achieve a high structural resolution of 0.295 nanometers — comparable to the one obtained with PAK4 complexes crystallized outside of the cell.
|
|
The research team also took advantage of the organization of this crystal. The PAK4 forms a hexagonal frame, similar to wax cells in a honeycomb. This hexagonal scaffolding has internal channels that can host other proteins. In the first instance, the research team fused Inka1 with a well-known fluorescent protein called GFP, allowing them to follow crystal growth with much more precision. Then, it turned out that almost any protein small enough, when fused to Inka1, can be crystallized inside the PAK4 honeycomb.
|
|
The team also monitored where and how quickly these crystals grew inside human cells. In most cases the crystals reached the full length of the cell cytoplasm — between 50 and 100 micrometers — before pausing. “Surprisingly cells tolerate the foreign crystal growing inside them and remain alive for days. Current spinoffs of this system include introducing environmentally responsive proteins into the crystals, which can make incredibly bright sensors,” concludes Manser.
|

