Nanocarbon: The Building Blocks of Advanced Carbon Nanomaterials
What is Nanocarbon?
Nanocarbon refers to various carbon-based materials with nanoscale dimensions, typically in the range of 1-100 nanometers. These materials exhibit unique physical, chemical, and electronic properties that differ from their bulk counterparts due to their small size and high surface-to-volume ratio. Nanocarbon materials serve as the fundamental building blocks for a wide range of advanced carbon nanomaterials and nanostructures.
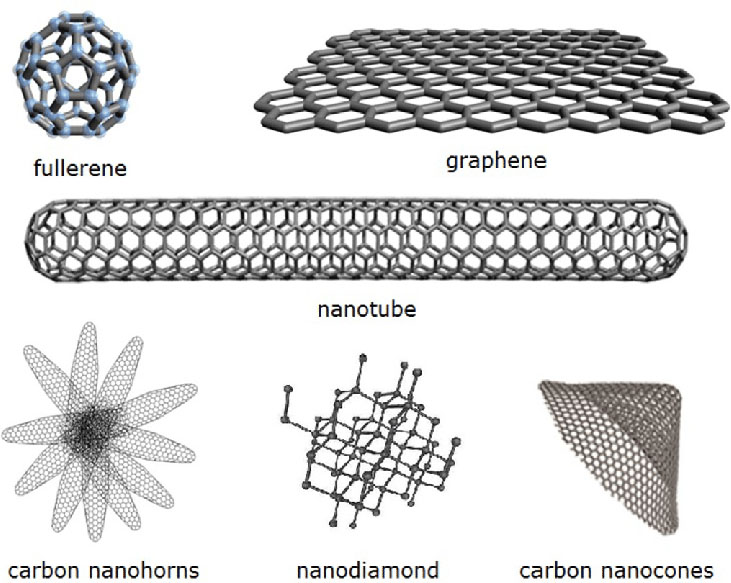
Types of Nanocarbon Materials
There are several main types of nanocarbon materials, each with distinct structures and properties:
Graphene
Graphene is a two-dimensional nanocarbon material consisting of a single layer of carbon atoms arranged in a hexagonal lattice. It is known for its exceptional mechanical strength, thermal conductivity, and electrical conductivity. Graphene serves as the basic structural element for other carbon nanomaterials, such as carbon nanotubes and graphite.
Carbon Nanotubes
Carbon nanotubes (CNTs) are cylindrical nanostructures formed by rolling up graphene sheets. They can be single-walled (SWCNTs) or multi-walled (MWCNTs), depending on the number of concentric graphene layers. CNTs exhibit extraordinary mechanical, thermal, and electrical properties, making them attractive for various applications, such as composites, electronics, and energy storage.
Fullerenes
Fullerenes are spherical or ellipsoidal nanocarbon structures composed of carbon atoms arranged in a closed cage-like structure. The most well-known fullerene is C60, also known as buckminsterfullerene, which consists of 60 carbon atoms in a soccer ball-like arrangement. Fullerenes have unique electronic and optical properties, making them promising for applications in solar cells, biomedical imaging, and drug delivery.
Nanodiamonds
Nanodiamonds are nanoscale particles of diamond, typically less than 10 nanometers in diameter. They are produced by detonation synthesis or high-pressure, high-temperature (HPHT) methods. Nanodiamonds possess the exceptional hardness and thermal conductivity of bulk diamond, along with unique surface properties and biocompatibility, making them suitable for various applications, such as abrasives, lubricants, and biomedical imaging.
Nanocarbon vs. Carbon Nanomaterial
While the terms "nanocarbon" and "carbon nanomaterial" are often used interchangeably, there is a subtle difference between them. Nanocarbon refers to the basic building blocks or structural units of carbon-based nanomaterials, such as graphene, CNTs, and fullerenes. On the other hand, carbon nanomaterials encompass a broader range of materials and structures that are composed of or derived from these nanocarbon building blocks.
Carbon nanomaterials can include more complex structures and composites, such as:
- Graphene Oxide (GO): A derivative of graphene with oxygen-containing functional groups on its surface, enhancing its dispersibility and chemical reactivity.
- Carbon Nanofibers (CNFs): Fibrous carbon nanomaterials with diameters in the range of 50-200 nanometers, often used in composites and energy storage applications.
- Carbon Quantum Dots (CQDs): Nanoscale carbon particles with sizes below 10 nanometers, exhibiting unique optical and electronic properties for bioimaging and sensing applications.
- Carbon Aerogels: Ultralight and highly porous carbon nanomaterials with a three-dimensional network structure, suitable for energy storage, catalysis, and environmental remediation.
In summary, nanocarbon materials serve as the fundamental building blocks, while carbon nanomaterials encompass a wider range of structures and composites derived from these basic units.
Synthesis and Production Methods
Various methods have been developed for the synthesis and production of nanocarbon materials, depending on the desired structure, properties, and scale of production. Some common methods include:
- Chemical Vapor Deposition (CVD): A versatile technique for growing graphene, CNTs, and other nanocarbon structures on substrates by decomposing carbon-containing precursors at high temperatures.
- Arc Discharge: A high-temperature method that involves the evaporation of graphite electrodes in an inert atmosphere, producing CNTs and fullerenes.
- Laser Ablation: A technique that uses high-energy laser pulses to vaporize a graphite target, resulting in the formation of CNTs and other nanocarbon structures.
- Liquid Phase Exfoliation: A scalable method for producing graphene and other 2D nanocarbon materials by exfoliating bulk graphite or other layered materials in liquid media using sonication or shear forces.
The choice of synthesis method depends on factors such as the desired nanocarbon structure, purity, yield, and production scale. Ongoing research focuses on developing more efficient, cost-effective, and environmentally friendly methods for the large-scale production of high-quality nanocarbon materials.
Applications of Nanocarbon Materials
Nanocarbon materials have found applications in a wide range of fields due to their exceptional properties and versatility. Some key application areas include:
- Electronics: Nanocarbon materials, particularly graphene and CNTs, are promising for next-generation electronic devices, such as transistors, sensors, and transparent conductive films.
- Energy Storage and Conversion: Nanocarbon materials are used in advanced batteries, supercapacitors, and fuel cells, offering high energy density, fast charge/discharge rates, and long cycle life.
- Composites: Incorporating nanocarbon materials into polymer, ceramic, or metal matrices can significantly enhance the mechanical, thermal, and electrical properties of the resulting composites.
- Biomedical Applications: Nanocarbon materials, such as graphene oxide and carbon quantum dots, show promise in biomedical applications, including drug delivery, bioimaging, and biosensing.
- Environmental Remediation: Nanocarbon materials, particularly carbon aerogels and activated carbon, are effective adsorbents for removing pollutants and contaminants from water and air.
As research advances, new applications of nanocarbon materials continue to emerge, leveraging their unique properties and synergies with other materials and technologies.
Challenges and Future Perspectives
Despite the tremendous progress in nanocarbon research and applications, several challenges remain to be addressed. One of the main challenges is the large-scale production of high-quality nanocarbon materials with consistent properties and minimal defects. The development of cost-effective and environmentally friendly production methods is crucial for the widespread adoption of nanocarbon materials in various industries.
Another challenge lies in understanding and controlling the structure-property relationships of nanocarbon materials. Fundamental research is needed to elucidate the mechanisms governing the growth, self-assembly, and functionalization of nanocarbon structures. Advanced characterization techniques and computational modeling tools will play a vital role in unraveling these complex relationships and guiding the rational design of nanocarbon materials with tailored properties.
Future research directions in nanocarbon materials include the development of multifunctional and smart nanocarbon-based systems, such as stimuli-responsive materials, self-healing composites, and bio-inspired structures. The integration of nanocarbon materials with other emerging technologies, such as 3D printing, flexible electronics, and energy harvesting, will open up new possibilities for innovative applications.
Furthermore, addressing the potential health and environmental risks associated with nanocarbon materials is crucial for their responsible development and deployment. Ongoing research efforts aim to understand the toxicity, biocompatibility, and environmental fate of nanocarbon materials, as well as to develop safe handling and disposal practices.
As the field of nanocarbon materials continues to evolve, interdisciplinary collaborations among materials scientists, chemists, physicists, engineers, and biologists will be essential to tackle the challenges and harness the full potential of these remarkable materials for a sustainable and technologically advanced future.
Further Reading
Nano Letters, Molecular Nanocarbon Science: Present and Future
