| Mar 26, 2020 | |
Novel hybrid nanocomposite sheds light on therapy of deep-seated orthotopic lung tumors(Nanowerk News) As a new type of 2D material, black phosphorus nanosheets (BPNSs) have attracted much attention owing to their unique 2D layered structure and layer-dependent bandgap of 0.3-2.0 eV. BPNSs have recently been adopted for photodynamic therapy (PDT), photothermal therapy (PTT), and drug delivery in theranostic nanomedicine. |
|
| However, the weak light absorption of BPNSs in the optically transparent window of biological tissues limits 1O2 generation and PTT efficiency in treating deep tumors. While combining with other nanomaterials, both PDT and PTT can theoretically achieve higher efficiency, this possibility has not yet been sufficiently explored. | |
| Recently, a research group led by prof. LIU Jun from Shanghai Institute of Optics and Fine Mechanics of the Chinese Academy of Sciences had made achievements on BPNSs combination with GNBPs (gold nanobipyramids) through plasmon excitation of improved 1O2 generation for PDT and localized hyperthermia for PTT. | |
| Their result was published in Acta Biomaterialia ("Gold nanobipyramid-loaded black phosphorus nanosheets for plasmon-enhanced photodynamic and photothermal therapy of deep-seated orthotopic lung tumors"). | |
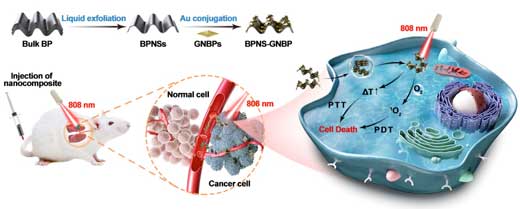 |
|
| Schematic illustration of the preparation of the BPNS-GNBP nanocomposite and LSPR-enhanced PDT-PTT. (Image: Shanghai Institute of Optics and Fine Mechanics) (click on image to enlarge) | |
| In the study, they developed a nanocomposite through the assembly of gold nanobipyramids (GNBPs) on black phosphorus nanosheets (BPNSs). This nanocomposite could simultaneously enhance 1O2 generation and hyperthermia by localized surface plasmon resonance in cancer therapy. | |
| As 2D inorganic photosensitizers, BPNSs were hybridized with GNBPs to form BPNS-GNBP hybrid nanosheets. The hybridization markedly increased 1O2 production by the BPNSs through plasmon-enhanced light absorption. The nanocomposite showed a higher photothermal conversion efficiency than the BPNSs alone. | |
| In vitro and in vivo assays indicated that the BPNS-GNBP hybrid nanocomposite exhibited good tumor inhibition efficacy owing to simultaneous dual-modality phototherapy. In vivo, the nanocomposite suppressed deep-seated tumor growth with minimal adverse effects in mice bearing orthotopic A549 human lung tumors. | |
| Taken together, these results demonstrate that BPNS-GNBP nanocomposite can function as a promising dual-modality phototherapeutic agent for enhanced cancer therapy in future cancer treatments. |
| Source: Chinese Academy of Sciences | |
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
