| Feb 22, 2023 |
Solvent affects structure of nano-coatings
(Nanowerk News) The choice of solvent used can control the specific structure of nanoparticle coatings, as has been demonstrated in a study headed by DESY NanoLab. The study shows that the solvent in which the nanoparticles are suspended before the coating is applied determines the way in which the particles arrange themselves in the resulting layer.
|
|
This means that the properties of the coating can be adapted to its intended use, as the team led by NanoLab director Andreas Stierle reports in the scientific journal Nanoscale ("Solvent controlled 2D structures of bottom-up fabricated nanoparticle superlattices"). This would allow the hardness of the coating to be optimised, for example.
|
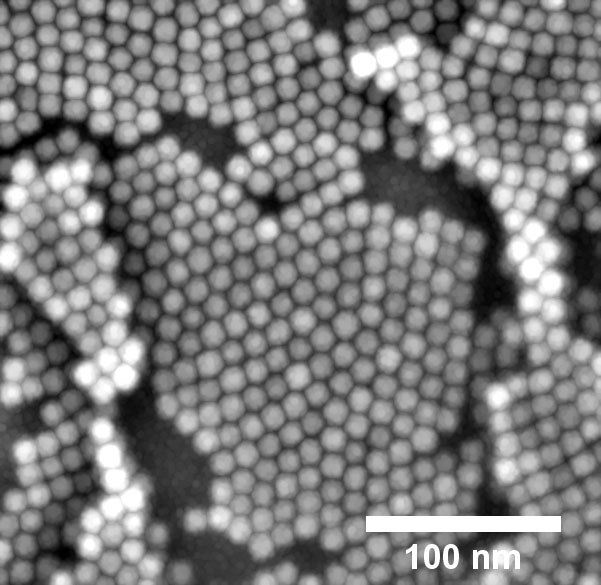 |
| When magnetite nanoparticles are sprayed with the solvent toluene, a hexagonal layer forms, as examination with the electron microscope shows. (Image: DESY, Arno Jeromin)
|
|
The researchers working at DESY, the University of Hamburg and the Hamburg University of Technology had investigated how nanocubes of magnetite (Fe3O4) arrange themselves during so-called spin coating. This process is used in the semiconductor industry among other places and allows very uniform layers to be applied to a substrate by spraying the coating liquid onto a rotating substrate. The rotational velocity and acceleration influence the distribution of the coating.
|
|
In this technique, nanoparticles have to be suspended in a solvent to be sprayed, which then evaporates during the coating process. The analysis using an scanning electron microscope shows that different structures are left behind, depending on the solvent used. “With the solvent toluene, for example, the nanocubes arrange themselves in a hexagonal pattern, whereas chloroform leads to a cubic arrangement,” explains Stierle. This structure has direct consequences for the properties of the coating. For example, the more densely packed hexagonal structure is usually harder than the cubic lattice.
|
|
“We believe that the different structures are already determined by the short-range configuration of the nanoparticles in the solvent,” says lead author Erik Beck from Stierle’s team. The nanoparticles are usually surrounded by a film of oleic acid. This standard treatment ensures that the particles bond better. “The different solvents interact with the oleic acid to different degrees,” Beck reports.
|
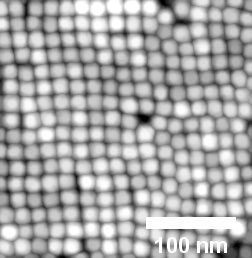 |
| When the solvent chloroform is used, the magnetite nanoparticles arrange themselves into a cubic layer. (Image: DESY, Arno Jeromin)
|
|
“The interaction is stronger in the case of chloroform, meaning that the oleic acid molecules spread out more and better mimic the shape of the nanoparticle. This creates a cubic layer,” explains Agnes Weimer from the University of Hamburg. “With toluene, on the other hand, the interaction is weaker, so the nanoparticles adopt a more spherical appearance in their oleic acid mantle, and form a hexagonal layer.”
|
|
The different structures are reminiscent of those formed by atoms in a crystal lattice, except that the nanoparticles are themselves tiny crystals. “This method of construction is typical of the way in which nature produces hard coatings, such as mother-of-pearl or dental enamel,” says Gerold Schneider from the Hamburg University of Technology. “Our method now means we are able to create coatings with specific desired properties.”
|
|
Together with colleagues from the University of Hamburg and the Hamburg University of Technology, the NanoLab team had already discovered in an earlier study that materials made of nanoparticles can be hardened by heating them. “Normally, such materials fracture along the oleic acid boundary layers,” explains Heshmat Noei from DESY NanoLab. “We observed that heating causes the oleic acid to react with oxygen in the air or in the nanomaterial, forming new organic compounds that are harder to break.” These experiments are published in the journal ACS Nano ("Strengthening Engineered Nanocrystal Three-Dimensional Superlattices via Ligand Conformation and Reactivity") and were also conducted with magnetite nanocubes.
|
|
“Usually, bulk materials are made from nanoparticles by gradually allowing the solvent to evaporate,” Stierle explains. “However, this is a relatively uncontrolled process. We were therefore looking for a process that would allow the material to grow in a well-ordered manner. With spin-coating, the material is built up layer by layer and can therefore be made to be particularly hard, for example. Making artificial dental enamel could be conceivable.”
|


