| Aug 07, 2020 | |
Probing interactions between MOFs and freestanding enzymes(Nanowerk News) Researchers have previously found that the biological functions of enzymes could be enhanced or otherwise altered – for instance by imparting new enzymatic functions – when they are encapsulated in metal-organic frameworks (MOFs). This is due to the interfacial interactions between the enzymes and the encapsulating MOF. |
|
| In new work, reported in Nano Letters ("Probing Interactions between Metal-Organic Frameworks and Freestanding Enzymes in a Hollow Structure"), researchers have probed the interactions of the enzyme catalase in solid and hollow ZIF-8 microcrystals. | |
| They found that the freestanding enzyme has less chemical interaction with ZIF-8 than confined catalase, and fluorescence study indicates that the freestanding catalase has lower structural confinement. | |
 |
|
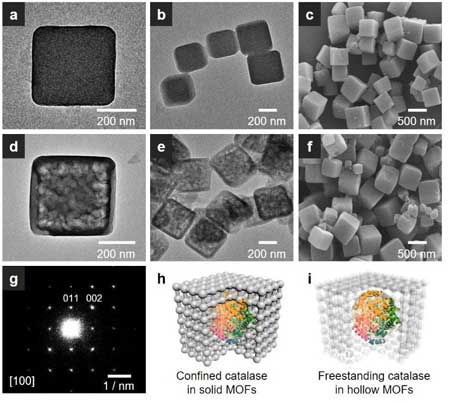
| TEM and SEM images of catalase encapsulated in (a-c) solid and (d-f) hollow MOFs. (g) The electron diffraction pattern of catalase in hollow ZIF-8. Schematic illustration of catalase encapsulated in (h) solid and (i) hollow MOFs. (Reprinted with permission by American Chemical Society) (click on image to enlarge) | |
| As illustrated in the figure above, before the hollowing process, the enzymes are confined in the solid MOFs crystals. After hollowing, the enzymes are sealed inside of the central cavity of hollow MOFs crystals in a freestanding form and the permeable MOFs shell allows reactants to go in without leaching of the enzymes. | |
| This hollowing strategy allows researchers to investigate the relationship between the interfacial interactions and biological function. This present study indicates that freestanding enzymes have less chemical interaction with the MOFs and that their structure is less confinement. | |
| The hollow structure reduces interfacial interactions and is inspired by the cell environment, such as glycolysis enzymes in the cytoplasm of living cells. | |
| "Our study not only highlights the importance of the freestanding state for the biological function of encapsulated enzymes but also presents a new way to immobilize freestanding enzymes in MOFs," the team concludes. |
 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
|
|
|
Subscribe to a free copy of one of our daily Nanowerk Newsletter Email Digests with a compilation of all of the day's news. |
