| Aug 14, 2014 |
Tissue development 'roadmap' created to guide stem cell medicine
|
|
(Nanowerk News) In a boon to stem cell research and regenerative medicine, scientists at Boston Children's Hospital, the Wyss Institute for Biologically Inspired Engineering at Harvard University and Boston University have created a computer algorithm called CellNet as a "roadmap" for cell and tissue engineering, to ensure that cells engineered in the lab have the same favorable properties as cells in our own bodies. CellNet and its application to stem cell engineering are described in two back-to-back papers in the August 14 issue of the journal Cell.
|
|
Scientists around the world are engaged in culturing pluripotent stem cells (capable of forming all the body's tissues) and transforming them into specialized cell types for use in research and regenerative medicine. Available as an Internet resource for any scientist to use, CellNet provides a much needed "quality assurance" measure for this work.
|
|
The two papers also clarify uncertainty around which methods are best for stem cell engineering, and should advance the use of cells derived from patient tissues to model disease, test potential drugs and use as treatments. For example, using CellNet, one of the studies unexpectedly found that skin cells can be converted into intestinal cells that were able to reverse colitis in a mouse model.
|
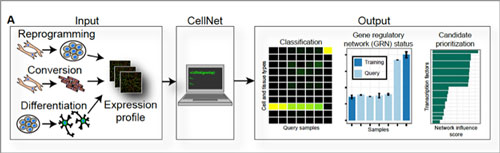 |
| Quality control for cells. CellNet is a computer algorithm that systematically assesses the quality of lab-engineered cells. The "inputs" are different kinds of engineered cells—induced pluripotent cells made through reprogramming techniques, specialized cells made through conversion of other specialized cells, and specialized cells made by coaxing a stem cell to differentiate. The "outputs" are comparisons of the lab-made cells' gene expression profiles to those of the real-life cells or tissues they are supposed to emulate (these "training" data are built into CellNet). At far right, CellNet flags and prioritizes potential genetic on/off switches (transcriptional regulators) that could be targeted to improve upon the engineered cells. (click on image to enlarge)
|
|
"To date, there has been no systematic means of assessing the fidelity of cellular engineering — to determine how closely cells made in a petri dish approximate natural tissues in the body," says George Q. Daley, MD, PhD, Director of the Stem Cell Transplantation Program at Boston Children's and senior investigator on both studies. "CellNet was developed to assess the quality of engineered cells and to identify ways to improve their performance."
|
|
Gene signatures
|
|
CellNet applies network biology to discover the complex network of genes that are turned on or off in an engineered cell, known as the cell's Gene Regulatory Network or GRN. It then compares that network to the cell's real-life counterpart in the body, as determined from public genome databases. Through this comparison, researchers can rigorously and reliably assess:
|
|
the quality of induced pluripotent stem cells (iPS cells) made by reprogramming blood cells or skin cells
the quality of specialized cells—such as liver, heart, muscle, brain or blood cells—made from either iPS cells or embryonic stem cells
the quality of specialized cells made from other specialized cells (such as liver cells made directly from skin cells)
what specific improvements need to be made to the engineering process.
|
|
"CellNet will also be a powerful tool to advance synthetic biology—to engineer cells for specific medical applications," says James Collins, PhD, Core Faculty member at the Wyss Institute and the William F. Warren Distinguished Professor at Boston University, co-senior investigator on one of the studies.
|
|
Putting CellNet to the test
|
|
The researchers—including co-first authors Patrick Cahan, PhD, and Samantha Morris, PhD, of Boston Children's, and Hu Li, PhD, of the Mayo Clinic, first used CellNet to assess the quality of eight kinds of cells created in 56 published studies.
|
|
In a second study, they applied CellNet's teachings to a recurring question in stem cell biology: Is it feasible to directly convert one specialized cell type to another, bypassing the laborious process of first creating an iPS cell? This study looked at two kinds of directly converted cells: liver cells made from skin cells, and macrophages made from B cells.
|
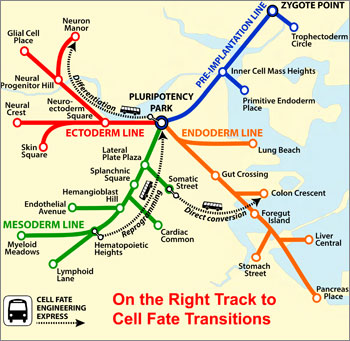 |
| CellNet maps the twists and turns of a cell's possible tissue "destinations," from its point of origin to the end of the line — as in the Boston subway system. (Image: Samantha Morris, PhD/Boston Children's Hospital)
|
|
"Most attempts to directly convert one specialized cell type to another have depended on a trial and error approach," notes Cahan, principle architect of CellNet and a post-doctoral scientist in the Daley lab. "Until now, quality control metrics for engineered cells have not gotten to the core defining features of a cell type."
|
|
In both test cases, CellNet showed that the engineered cells hadn't completely converted, retaining some characteristics of their cells of origin—but pointed to specific genetic tweaks that could be done in the lab to fix the problem.
|
|
CellNet also pointed out some useful properties that weren't apparent before. "We found that liver-like cells made from mouse skin were actually more like intestinal cells," says Morris. "In fact, the converted skin cells could engraft into mice with inflammatory bowel disease—Crohn's or ulcerative colitis. After a short time, the cells became highly similar to native colon cells and assisted healing of the damaged tissue, a finding that surprised and excited us."
|
|
Guidance for stem cell engineering
|
|
Together, the two studies establish some general principles for stem cell science:
|
|
1) The GRN of iPS cells created by reprogramming a mature cell is nearly identical to that of stem cells made from embryos, confirming that iPS cells are a good raw material for creating specialized cells.
|
|
2) Once engineered cells are engrafted into laboratory mice, their GRN becomes even closer to that of the true target tissue, indicating that the body's own tissues contribute signals to enhance the performance of transplanted cells.
|
|
3) Differentiating pluripotent stem cells into specific tissues is currently more effective than attempting to convert one specialized cell directly to another, creating cells whose GRNs are much like those of cells in the body.
|
|
4) Most specialized cells made from other specialized cells retain some "memory" of their cell of origin, making them less than ideal for certain uses but better for others.
|


