Single-Molecule Localization Microscopy: Surpassing the Diffraction Limit for Nanoscale Imaging
What is Single-Molecule Localization Microscopy?
Single-molecule localization microscopy (SMLM) is a super-resolution imaging technique that enables the visualization of biological structures and processes at the nanoscale. By precisely localizing individual fluorescent molecules, SMLM overcomes the diffraction limit of conventional light microscopy, allowing researchers to observe cellular components and dynamics with unprecedented detail.
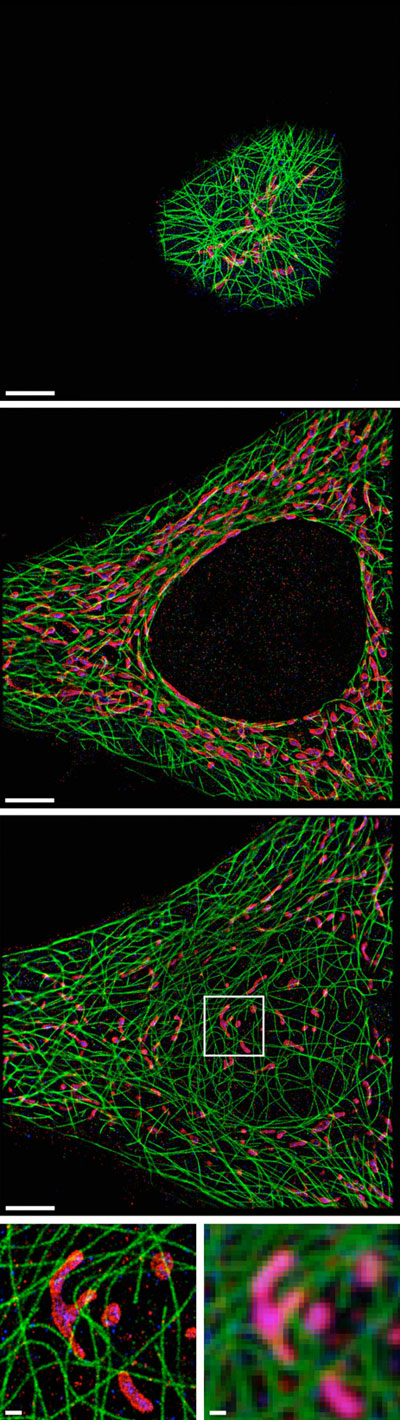
Principles of SMLM
SMLM relies on the stochastic activation and precise localization of individual fluorescent molecules to achieve super-resolution:
- Stochastic Activation: Fluorescent molecules are switched between a dark state and a fluorescent state in a stochastic manner. This ensures that only a sparse subset of molecules is active at any given time, allowing for their individual detection and localization.
- Precise Localization: The position of each fluorescent molecule is determined with high precision by fitting its point spread function (PSF) to a mathematical model, typically a 2D Gaussian function. This localization process is repeated over thousands of imaging frames, with each frame capturing a different subset of active molecules.
- Image Reconstruction: The localization data from all frames are combined to reconstruct a super-resolved image, where each molecule is represented by a single point at its estimated position. This results in a highly detailed map of the fluorescently labeled structures, revealing features that are otherwise hidden in conventional microscopy.
SMLM Techniques
Several SMLM techniques have been developed, each with its own strengths and applications:
Photoactivated Localization Microscopy (PALM)
PALM utilizes photoactivatable fluorescent proteins, which can be switched from a dark state to a fluorescent state upon exposure to a specific wavelength of light. By controlling the activation of these proteins, PALM achieves single-molecule localization and super-resolution imaging of genetically labeled structures in live cells.
Stochastic Optical Reconstruction Microscopy (STORM)
STORM employs photoswitchable dyes, which can be reversibly switched between a fluorescent state and a dark state. By controlling the switching of these dyes with light of different wavelengths, STORM enables the localization and imaging of immunolabeled structures with high specificity and resolution.
DNA-PAINT
DNA-PAINT (Points Accumulation for Imaging in Nanoscale Topography) uses transient binding of fluorescently labeled DNA probes to their complementary targets on the sample. By repeatedly localizing these transient binding events, DNA-PAINT achieves high-resolution imaging without the need for photoswitching or photobleaching of fluorophores.
Comparison with Other Super-Resolution Techniques
SMLM differs from other super-resolution techniques in several key aspects:
- Structured Illumination Microscopy (SIM): SIM uses patterned illumination to encode high-frequency information into the sample, which is then computationally reconstructed to achieve a two-fold improvement in resolution. While SIM offers faster imaging speeds and lower phototoxicity compared to SMLM, it has a lower resolution enhancement and is more sensitive to sample-induced aberrations.
- Stimulated Emission Depletion (STED) Microscopy: STED uses a doughnut-shaped depletion beam to confine the fluorescence emission to a sub-diffraction volume, enabling super-resolution imaging. STED provides a continuous resolution improvement and faster imaging speeds compared to SMLM but requires higher laser intensities and is limited by the availability of suitable fluorophores.
- Expansion Microscopy (ExM): ExM physically expands the sample by embedding it in a swellable polymer matrix, allowing for super-resolution imaging with conventional microscopes. While ExM offers a simple and accessible approach to super-resolution, it requires sample preparation steps that may introduce artifacts and is not suitable for live-cell imaging.
SMLM stands out for its ability to achieve the highest spatial resolution among these techniques, often reaching a localization precision of 10-20 nm. However, it typically requires longer imaging times and specialized fluorophores, making it more suitable for fixed samples and slower biological processes.
Applications of SMLM
SMLM has revolutionized the study of biological systems at the nanoscale, enabling groundbreaking discoveries in various fields:
- Cellular Imaging: SMLM allows for the visualization of subcellular structures, such as the cytoskeleton, organelles, and protein complexes, with unprecedented detail. This has led to new insights into cell morphology, organization, and function.
- Molecular Interactions: By imaging multiple targets labeled with different fluorophores, SMLM enables the study of molecular interactions and co-localization at the nanoscale. This has been particularly useful in investigating receptor clustering, signaling complexes, and protein-protein interactions.
- Structural Biology: SMLM has been applied to study the structure and organization of macromolecular complexes, such as nuclear pore complexes, centrosomes, and viral particles. By combining SMLM with other techniques, such as electron microscopy, researchers can obtain a comprehensive understanding of these structures.
- Neuroscience: SMLM has been used to map the nanoscale organization of synapses, revealing the distribution and dynamics of neurotransmitter receptors, synaptic vesicles, and other key components. This has provided new insights into synaptic function, plasticity, and neurological disorders.
As SMLM continues to evolve, with improved labeling strategies, faster imaging speeds, and integration with other techniques, it holds great promise for unraveling the complexities of biological systems at the nanoscale.
Further Reading
Physical Chemistry Chemical Physics, Single-Molecule Localization Microscopy - near-molecular spatial resolution in light microscopy with photoswitchable fluorophores
Nature Reviews Methods Primers, Single-Molecule Localization Microscopy
Universität Würzburg, Doctoral Thesis, Advancing Single-Molecule Localization Microscopy: Quantitative Analyses and Photometric Three-Dimensional Imaging
