STED Microscopy: Revolutionizing Nanoscale Imaging in Biology and Beyond
What is STED Microscopy?
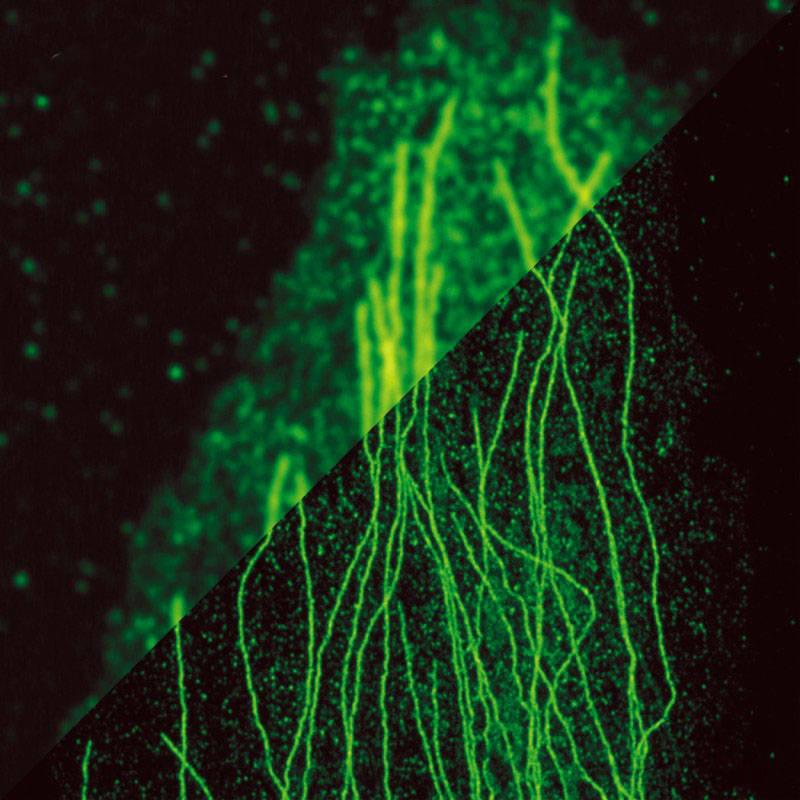
Stimulated Emission Depletion (STED) microscopy is a super-resolution imaging technique that overcomes the diffraction limit of conventional optical microscopy. It enables the visualization of nanoscale structures and processes with unprecedented spatial resolution, typically in the range of 20-50 nanometers. STED microscopy relies on the selective deactivation of fluorophores to achieve superior resolution and contrast.
Key Principles of STED Microscopy
STED microscopy is based on two key principles: fluorescence excitation and stimulated emission depletion.
Fluorescence Excitation
In STED microscopy, the sample is labeled with fluorescent dyes or probes that can be excited by a specific wavelength of light. When excited, these fluorophores emit light at a longer wavelength, which is detected to form the image. The excitation is typically performed using a focused laser beam.
Stimulated Emission Depletion
The core principle of STED microscopy is the selective deactivation of fluorophores using stimulated emission. A second laser, called the STED laser, is used to create a donut-shaped intensity profile that overlaps with the excitation laser. The STED laser has a wavelength that matches the emission spectrum of the fluorophores.
When the STED laser is applied, it induces stimulated emission in the fluorophores, causing them to rapidly return to the ground state without emitting fluorescence. This effectively "turns off" the fluorophores in the donut-shaped region, leaving only a small central area where fluorescence can occur.
By scanning this small fluorescent spot across the sample, a super-resolution image is obtained, as the effective resolution is determined by the size of the remaining fluorescent area rather than the diffraction limit.
Advantages of STED Microscopy
STED microscopy offers several advantages over conventional optical microscopy:
- Super-Resolution Imaging: STED microscopy provides spatial resolution well beyond the diffraction limit, enabling the visualization of nanoscale structures and processes that were previously unresolvable with optical techniques.
- Live-Cell Imaging: STED microscopy is compatible with live-cell imaging, allowing researchers to study dynamic processes in real-time with high resolution. This is particularly valuable for investigating cellular structures, protein interactions, and molecular dynamics.
- Multicolor Imaging: STED microscopy can be extended to multicolor imaging by using different fluorophores and corresponding excitation and STED lasers. This enables the simultaneous visualization of multiple cellular components or molecular species with high resolution.
- Combinability with Other Techniques: STED microscopy can be combined with other imaging modalities, such as fluorescence correlation spectroscopy (FCS) or fluorescence lifetime imaging (FLIM), to extract additional information about the sample, such as molecular dynamics or physicochemical properties.
Comparison with Other Super-Resolution Techniques
STED microscopy is one of several super-resolution imaging techniques that have emerged in recent years. Other notable techniques include structured illumination microscopy (SIM) and single-molecule localization microscopy (SMLM), such as photoactivated localization microscopy (PALM) and stochastic optical reconstruction microscopy (STORM). Each technique has its own strengths and limitations, and the choice of method often depends on the specific research question and sample characteristics.
Structured Illumination Microscopy (SIM)
SIM achieves super-resolution by illuminating the sample with patterned light and computationally reconstructing the high-resolution image from multiple low-resolution images. SIM typically provides a resolution improvement of about 2-fold compared to conventional microscopy. While SIM offers faster imaging speeds and lower phototoxicity compared to STED, its resolution enhancement is more limited.
Single-Molecule Localization Microscopy (SMLM)
SMLM techniques, such as PALM and STORM, rely on the precise localization of individual fluorophores over multiple imaging cycles. By stochastically activating and localizing a subset of fluorophores in each cycle, a super-resolution image is reconstructed. SMLM can achieve resolutions down to 10-20 nanometers, surpassing STED. However, SMLM typically requires longer acquisition times and specialized fluorophores, and is more challenging for live-cell imaging due to the need for multiple imaging cycles.
In comparison, STED microscopy offers a balance of high spatial resolution (20-50 nanometers), relatively fast imaging speeds, and compatibility with live-cell imaging. STED is particularly well-suited for studying dynamic processes and structures in real-time, such as protein trafficking or cytoskeletal rearrangements.
Researchers often choose STED microscopy when they require high-resolution imaging of specific cellular structures or processes in living cells. However, STED can also be combined with other super-resolution techniques, such as SMLM, to provide complementary information and further enhance the spatial resolution.
Applications of STED Microscopy
STED microscopy has found numerous applications in various fields, including:
Cell Biology
STED microscopy has revolutionized the study of cellular structures and processes at the nanoscale. It has been used to visualize the organization of proteins in cell membranes, the structure of organelles like mitochondria and endoplasmic reticulum, and the dynamics of molecular interactions within cells.
Neuroscience
STED microscopy has greatly contributed to the field of neuroscience by enabling the high-resolution imaging of neuronal structures, such as synapses, dendritic spines, and axonal projections. It has shed light on the molecular organization and plasticity of synapses, which is crucial for understanding brain function and disorders.
Virology and Immunology
STED microscopy has been applied to study viral infection processes and the immune response at the nanoscale. It has allowed researchers to visualize the entry, replication, and assembly of viruses within host cells, as well as the interactions between immune cells and pathogens.
Challenges and Future Perspectives
Despite its remarkable capabilities, STED microscopy also faces some challenges. One major challenge is the potential for photobleaching and phototoxicity due to the high laser intensities used for STED. This can limit the imaging duration and affect the viability of live samples. Researchers are actively developing strategies to minimize these effects, such as using photostable fluorophores and optimizing imaging conditions.
Another challenge is the complexity of STED microscopy systems, which require precise alignment and synchronization of multiple lasers and optical components. Ongoing efforts are focused on simplifying the instrumentation and making STED microscopy more accessible to a wider range of users.
Future developments in STED microscopy aim to further improve the resolution, speed, and depth of imaging. Researchers are exploring the use of adaptive optics to correct for aberrations in thick samples, as well as the integration of STED with other super-resolution techniques like single-molecule localization microscopy (SMLM) to achieve even higher resolution.
Additionally, the development of new fluorescent probes and labeling strategies specifically tailored for STED microscopy will expand its applicability to a broader range of biological systems and research questions.
Further Reading
Chemical Reviews, Stimulated Emission Depletion Microscopy
Microscopy Research and Technique, Stimulated emission depletion microscopy for biological imaging in four dimensions: A review
Measurement Science and Technology, Progresses in implementation of STED microscopy
