| Feb 24, 2022 |
Utilizing carbon dots in electrochemical processes and energy storage
(Nanowerk News) Thanks to their unusual optical properties, carbon particles with diameters on the order of a few nanometers – so-called carbon dots – show great promise for a wide range of technological applications, as diverse as energy conversion and bio-imaging.
|
|
Moreover, carbon dots (CDs) have several practical advantages as they are easy to fabricate, stable and contain no toxic heavy metals. Their versatility is largely due to the fact that, depending on their chemical composition and aspects of their complex structure, they can either act as emitters of light in the form of photoluminescence or function as photocatalysts by absorbing light energy and triggering chemical reactions, such as water splitting.
|
|
Furthermore, their superior electrochemical activity and ease-of-modification make carbon dots very promising electrode materials in electrocatalysis and electrical energy storage.
|
|
The structure of CDs usually consists of a core composed of sp2/sp3 hybrid carbon atoms and an amorphous shell with plentiful O/N-containing functional groups or polymer chains. According to the microstructure of carbon cores, CDs are further divided into graphene quantum dots (GQDs), carbon quantum dots (CQDs), carbon nanodots (CNDs) and carbonized polymer dots (CPDs)
|
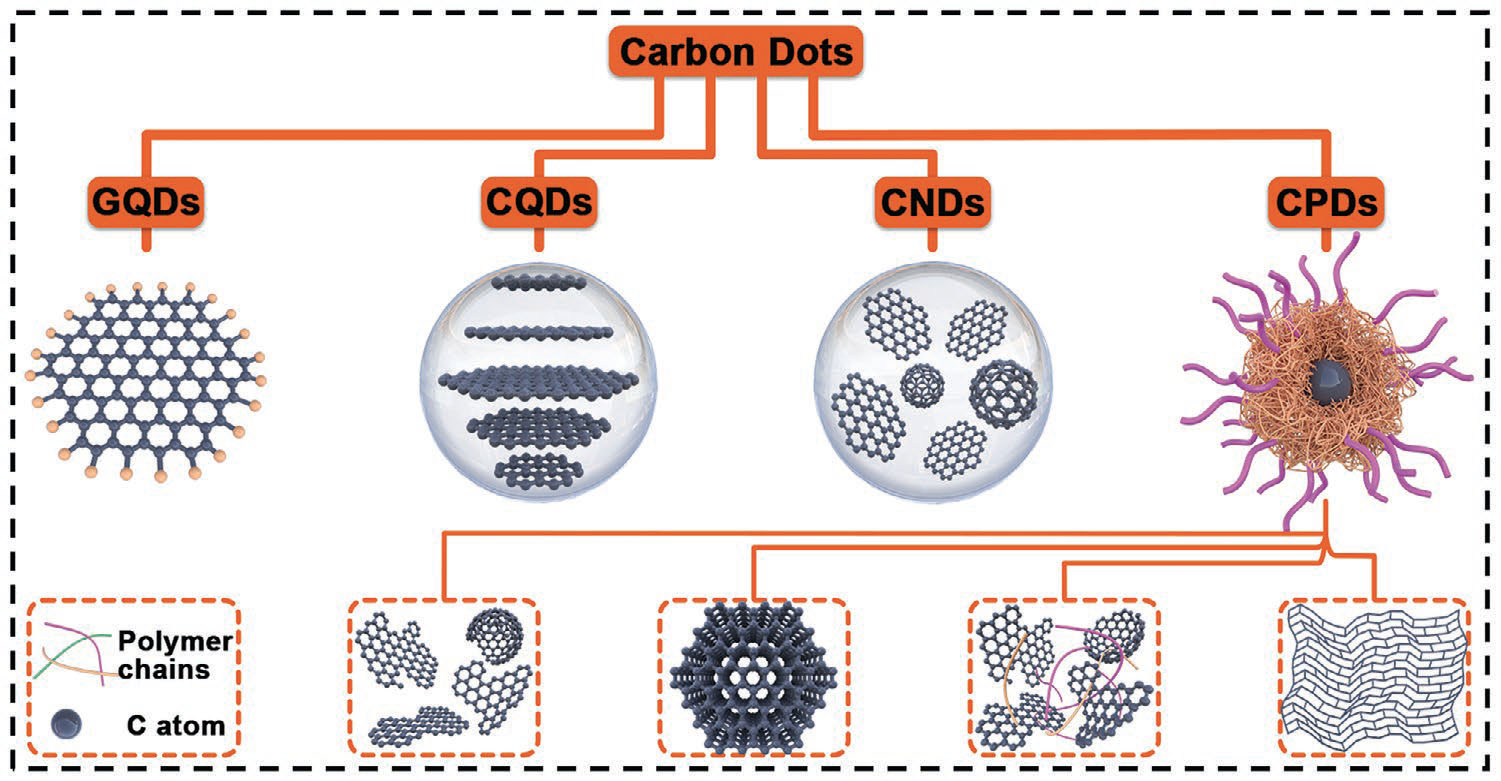 |
| Four categories of carbon dots and their structures: graphene quantum dots (GQDs), carbon quantum dots (CQDs), carbon nanodots (CNDs), and carbonized polymer dot (CPDs). (Reproduced with permission by Wiley-VCH Verlag)
|
|
A review in Advanced Energy Materials ("Carbon Dots as New Building Blocks for Electrochemical Energy Storage and Electrocatalysis"), summarizes recent advances in CDs-based electrode materials, including synthesis methods, structure and physiochemical properties of CDs, as well as modification and functionalization strategies, with particular emphasis on structure-property relationships.
|
|
The article also discusses applications of CDs-containing electrodes in H2/O2 evolution, O2 reduction, CO2 reduction, capacitors, and batteries.
|
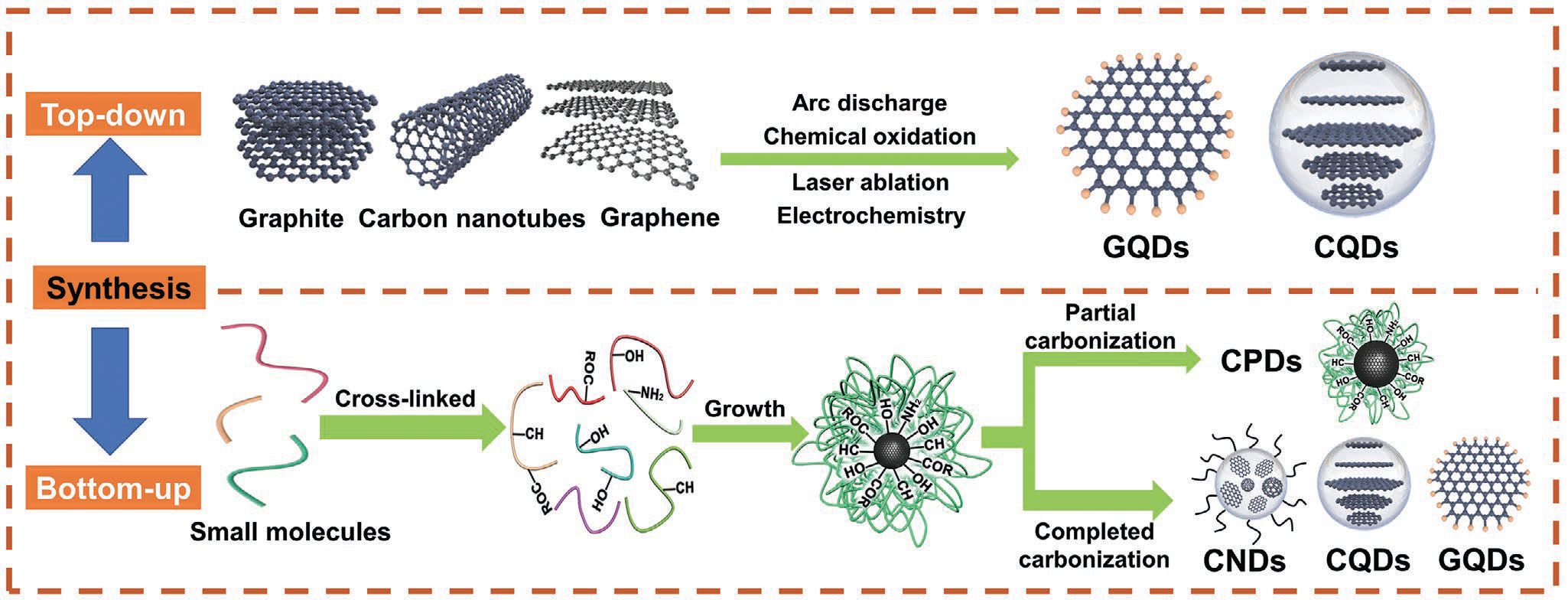 |
| Schematic illustration of the synthesis of CDs via top-down and bottom-up methods. (Reproduced with permission by Elsevier)
|
|
CDs, because of their unique structural and physiochemical characteristics, are considered to be promising electrode candidates for supercapacitors and batteries. In their article, the authors therefore summarize recent research in this area, highlighting the advantages of CDs in such devices. They address water splitting to produce H2 and CO2 reduction to fuels over CDs-containing electrodes, which are also effective technologies for storing electrical energy.
|
|
Although plenty of encouraging results have been achieved to date, research into CDs-based electrode materials is still in its nascent stage. This research focuses on five key functions of applying carbon dots in electrochemical energy storage and conversion through diverse processes:
|
|
As active centers to provide more electrochemically active sites through inherent structural defects, abundant surface/edge functional groups, and heteroatom doping;
As regulators to adjust the electronic state and local charge distribution of active centers in other catalytic materials, thereby changing the adsorption ability of the catalyst to reaction intermediates;
As structure stabilizers to anchor and disperse active components through abundant surface functional groups, to reduce the agglomeration of active materials by forming a restricted network, and to alleviate volume changes during charge–discharge process by forming protective/buffer layers;
As structure-directing agents to induce the crystallization and the growth of metal nanomaterials to form electrochemically-beneficial morphologies and microstructures;
As conductive agents to boost the kinetics of ion diffusion and charge transfer.
|
|
As this review illustrates, CDs-based materials show unique advantages and great potential in electrochemical energy conversion and storage systems. The authors conclude that, with the discovery of advanced synthesis and characterization methods and deep comprehension of the relationships between CDs structure and performance, the rational design and construction of high-performance CD-based electrodes will be achieved in the near future, paving the way for the commercialization of a host of CD electrode-containing energy storage and conversion devices.
|


 By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
By
Michael
Berger
– Michael is author of three books by the Royal Society of Chemistry:
Nano-Society: Pushing the Boundaries of Technology,
Nanotechnology: The Future is Tiny, and
Nanoengineering: The Skills and Tools Making Technology Invisible
Copyright ©
Nanowerk LLC
