| Posted: August 19, 2009 |
Superior cathode material for electrochemical energy storage devices |
|
(Nanowerk News) Amongst all the commercially available power sources, lithium-ion batteries (LIBs) currently represent the state-of-the-art technology in high energy batteries, and has occupied a prime position in the market place to power portable electronic devices such as, laptops, personal digital assistants, and cellular phones. However, for the use as power supplies of electric vehicles (EVs) and hybrid electric vehicles (HEVs), it is still a challenge for LIBs to achieve long-term cycling life and high power density in which supercapacitors currently address the extremes.
|
|
Recently, with the financial supports of the Chinese Academy of Science, MOST and the National Natural Science Foundation, researchers of the CAS Key Laboratory of Molecular Nanostructure and Nanotechnology successfully designed and synthesized a nanocomposite of LiFePO4 nanoparticles embedded in nanoporous carbon matrix (LFP-NP@NPCM) as a superior cathode material for LIBs. This work has been published in the recent issue of Advanced Materials ("LiFePO4 Nanoparticles Embedded in a Nanoporous Carbon Matrix: Superior Cathode Material for Electrochemical Energy-Storage Devices").
|
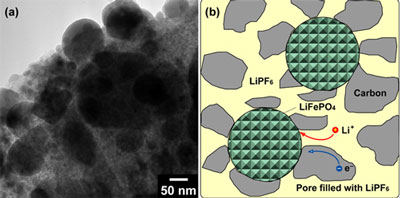 |
| Figure 1. (a) TEM image and (b) schematic illustrations of the LFP-NP@NPCM nanocomposite.
|
|
Compared with the commercial LiCoO2, olivine-structural LiFePO4 has attracted extensive interest as a potential cathode material for LIBs because of its numerous appealing features such as high theoretical capacity (170 mA h g-1), high safety, environmental benignity, and low cost. One of the challenging issues in using it for high power LIBs is to tackle its sluggish mass and charge transport. In the present LFP-NP@NPCM, nanometer-sized LiFePO4 particles uniformly embed in the nanoporous carbon matrix (Fig. 1), which own the following virtues: i) Nanometer-sized LiFePO4 particles could decrease the Li diffusion distance and time, resulting in much improved power capability; ii) The pores in the porous carbon matrix serve as electrolyte-containers for high rate charge/discharge process; iii) The carbon matrix enhances the electronic conductivity of nanocomposite; iv) The carbon matrix stabilizes the nanoscaled LiFePO4, and then improves the cycling performance.
|
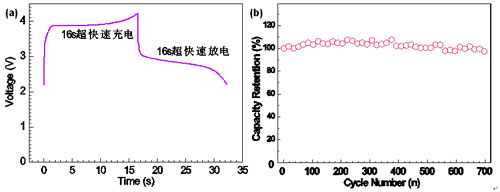 |
| Figure 2. (a) Ultrafast charge/discharge curves of the LFP-NP@NPCM nanocomposite; (b) Cycling performance of the LFP-NP@NPCM nanocomposite cycled at a rate of 1.5 C
|
|
Therefore, the LFP-NP@NPCM electrode can be fully charged or discharged within a period of about 16 s (Fig. 2a), similar to a supercapacitor, but with more energy density. Another excellent property of the LFP-NP@NPCM nanocomposite is the superior cycling performance. The discharge capacity loss is less than 3% over 700 cycles at a rate of 1.5C (Fig. 2b).
|


