| Posted: September 10, 2009 |
Novel nanotechnology drug delivery tool works with nanoparticles and lasers |
|
(Nanowerk News) Researchers at UC Santa Barbara have developed a new way to deliver drugs into cancer cells by exposing them briefly to a non-harmful laser. Their results are published in a recent article in ACS Nano, a journal of the American Chemical Society.
|
|
"This entirely novel tool will allow biologists to investigate how genes function by providing them with temporal and spatial control over when a gene is turned on or off," explained Norbert Reich, senior author and a professor in the Department of Chemistry and Biochemistry at UCSB. "In a nutshell, what we describe is the ability to control genes in cells –– and we are working on doing this in animals –– simply by briefly exposing them to a non-harmful laser."
|
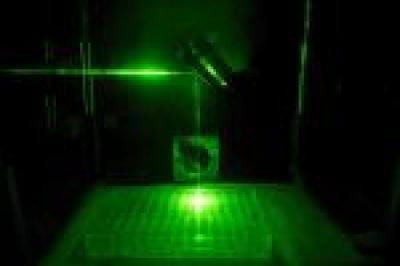 |
| The near-infrared laser pathway into the cell culture plate, traced by visible laser for photo. (Image: Rod Rolle)
|
| The scientists used cancer cells from mice, and grew them in culture. They then introduced gold nanoshells, with a peptide-lipid coating, that encapsulated "silencing ribonucleic acid" (siRNA), which was the drug that was taken up by the cells. Next, they exposed the cells to a non-harmful infrared laser.
|
|
"A major technical hurdle is how to combine multiple biochemical components into a compact nanoparticle which may be taken up by cells and exist stably until the release is desired," said Gary Braun, first author and a graduate student in UCSB's Department of Chemistry and Biochemistry. "Laser-controlled release is a convenient and powerful tool, allowing precise dosing of particular cells within a group. The use of biologically friendly tissue penetration with near-infrared light is the ideal for extending this capability into larger biological systems such as tissues and animals."
|
|
The authors demonstrated, for the first time, the delivery of a potent siRNA cargo inside mammalian cancer cells, released by exposing the internalized nanoparticles for several seconds to a pulsed near-infrared laser tuned for peak absorption with a specific spatial pattern. The technique can be expanded to deliver numerous drug molecules against diverse biological targets.
|

