| Posted: September 19, 2008 |
A metal catalyst teaches an old chemical reaction new tricks |
|
(Nanowerk News) A new twist on a common chemical reaction has enabled RIKEN scientists to create molecules that are useful building blocks for making new pharmaceuticals.
|
|
The aldol reaction is a very reliable way to stitch together two carbon-based organic molecules that each contain a carbonyl group—a carbon atom doubly bonded to an oxygen atom.
|
|
Organic chemists have devoted enormous effort to developing asymmetric forms of this reaction. The products of these reactions cannot be superimposed on their ‘mirror image’ molecule—just as your left hand cannot be superimposed on your right.
|
|
Nature often selects molecules of a particular handedness; so many pharmaceuticals must be the correct mirror image form. But it is often difficult to synthesize a chemical of one handedness without also producing an equal amount of its twin.
|
|
Pilot molecules are often added to these reactions to steer their progress, ensuring that the correct form of the desired chemical is produced. Ideally, these molecules should speed up the reaction even though they are present in only trace amounts, and should not be consumed by the reaction. Chemists call them asymmetric catalysts.
|
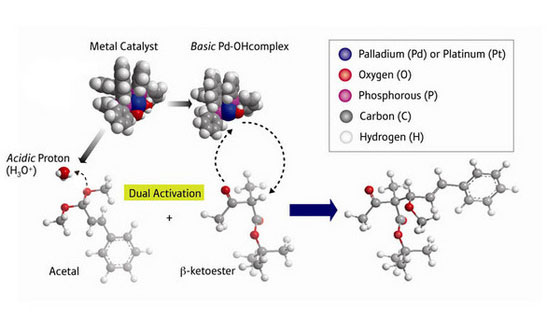 |
| An asymmetric aldol-type reaction catalyzed by a palladium acid-base catalyst. (Image: RIKEN)
|
|
Mikiko Sodeoka and colleagues at RIKEN’s Advanced Science Institute in Wako have now developed an asymmetric catalyst that connects two different types of molecular building blocks—β-ketoesters and acetals—in an aldol reaction to form new compounds of a single handedness ("Catalytic Enantioselective Aldol-type Reaction of β-Ketosters with Acetals"). “This type of aldol reaction has long been recognized to be notoriously difficult, and the key to success is the double activation by the Pd complex as an acid-base catalyst,” says Sodeoka.
|
|
The catalyst is made from the metal palladium, attached to a bulky molecule called binap which has a twist in its structure. This twist ensures that the β-ketoester molecule sticks to the central palladium metal atom in a specific way—which, in turn, determines the orientation of the acetal connecting to it.
|
|
Sodeoka and her team tried a range of different β-ketoester and acetal combinations, and found that in most cases the catalyst helped to form the product in almost entirely the correct handedness. The compounds can in principle be converted into important building blocks for many drugs, which would otherwise be difficult to make catalytically using conventional methods.
|
|
The team also developed an alternative catalyst, which used platinum in place of palladium, and found that it was more resistant to decomposition in those reactions which took a long time to complete. They now hope to refine the catalysts' design, and use them to create more complex, bioactive molecules.
|

